Team:Paris Bettencourt/Designs
From 2011.igem.org

Introduction
This page is a summing up of the design we have builded and explain the ideas that are behind the designs. Do not start reading this page if you have not read the project overview first.
Testing the nanotubes with simple experiments
In the paper from Dubey and Ben Yehuda, many types of experiments where done to demonstrate the existence and the efficiency of the nanotube as a mean of communication between bacteria. We wanted to do again some experiments. First to be sure we are able to put ourself in the conditions where the nanotubes exists, in our own laboratory. Second, we wanted to verify some results that are the object of many discussion in the community.
In these experiments, there are no amplification system. We simply obeserve, directly and indirectly the molecules that have passed through the tubes.
Here is the list of the experiments:
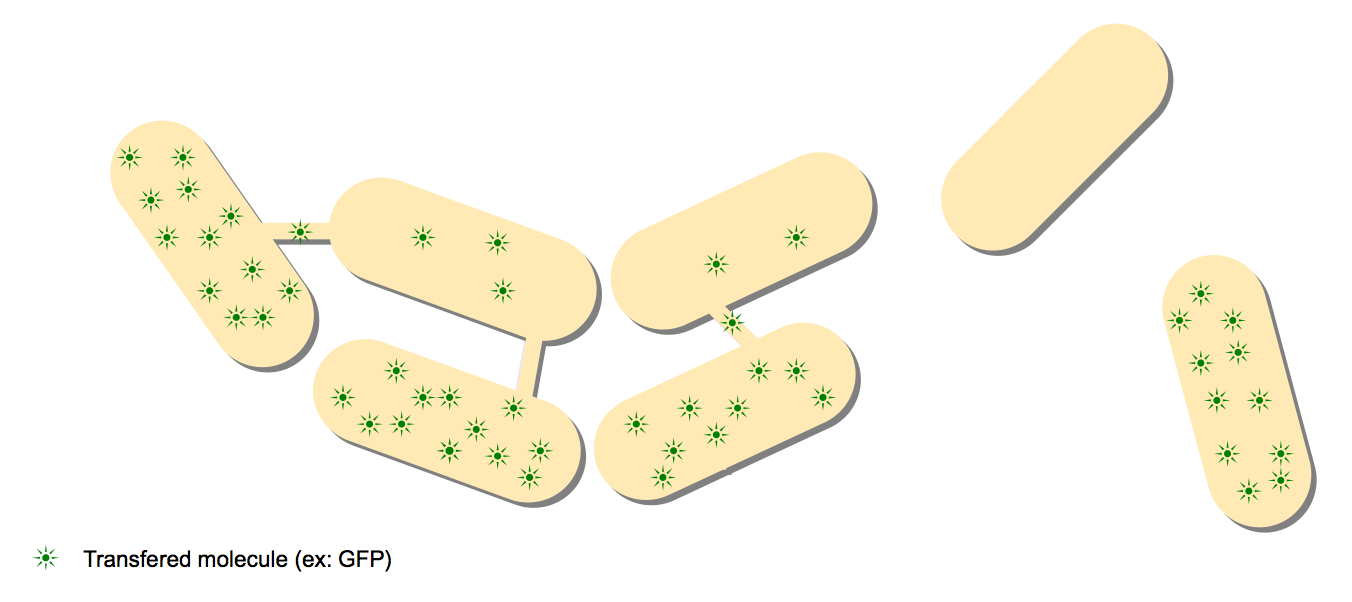
- The GFP diffusion experiment: This experiment is the keystone experiment of the paper. One strain expressing GFP is mixed with one wild type strain, and the GFP proteins are diffusing from the GFP cells to the colorless cells.
- The YFP-TetR/TetO array experiment: This experiment is an improvement of the previous one. To observe significant fluorescence in the neighboring cell, lots of molecule have to pass through the tubes. Using the affinity of the TetR for the TetO array, we want to concentrate at one point the GFP molecules to see them better with a smaller number of molecules.
- The antibiotics resistance exchange experiment: This experiment is the most controversed experiment of the paper. The author claims that a mix of two strains, each of them holding a resistance for a given antibiotic can survive together in a medium containig the two antibiotics because they exchange resistance enzymes through the nanotubes. We found that there are other possible explanation for this experiment than the existence of the nanotube.
Designs for nanotube characterization
Key specification of our designs
The aim of our project is, using the techniques of synthetic biology, to characterize and control the cellular communication through the nanotubes. At the origin, synthetic biology was not an engeenering question. The idea was to build artificial systems to explore more deeply the properties of the cells and the organism. Our project is clearly following this idea
The first question about the nanotube we asked ourself is what kind of molecules can we pass through the nanotubes. Have them to be big? Have them to be very small? The paper shows that a GFP can pass, but what is the protein is bigger?
The second question we asked ourself was the process that are happening inside the tubes. Is it simple diffusion? Is there an active process?
We started designing the project these two questions in mind. Trying to pass through the tubes several molecules of very different size, from a T7 polymerase to a tRNA amber supressor, we aim to test the capability and size limit for the molecules that can pass through the tubes. But, if the diffusion is passive, the speed of diffusion should be proportional to the diffusion coefficient D, that is to say invertially proportinal to the radius of the object. Can we design system with fast response time that would allow us to measure this time?
Now the concept are set, we builded a general requirement map for the design. This can be simply summed up by this schematic: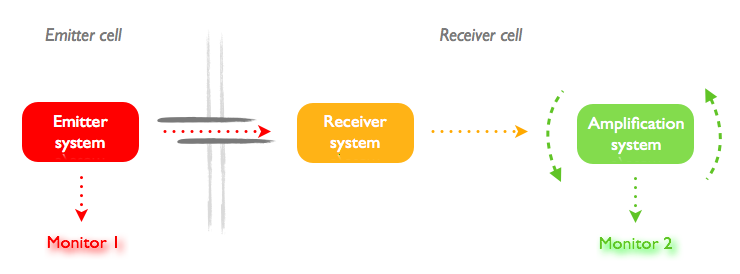
The problem is that each design needs a different couple of signal emittor/receptor. The problem is even with a good modelisation, we cannot estimate the response time of these element. We tried to turn around this priblem.
- First, we try to use each time the same actuator and the same monitor to have comparable response times in beetween the systrems.
- Second, we will not compare the time itself, but the increase of response time for the emittor and receptor system placed in the same cell of in two different cells, linked by the tube. This increase of delay is expected to be only due to the diffusion through the tube.
Inter-species communication
The article demonstrate that nanotubes established inside the B. Subtilis familly, but can also be established between an B. Subtilis and an E. Coli. Theses two type of bacteria are really different since one is Gramm+ and the other is Gramm-. Their membrane is really different, since there is no periplasm in B. Subtilis. The existence of interspecies nanotube did really intrigate us and we wanted also to buil designs to test this aspect of the communication.
B. Subtilis is Gramm-. The nanotube is known tu create a jonction from the cytoplasm of the first cell to the cytoplasm of the second one. In the case of the Gramm+ bacteria, like E. Coli, we wonder toward which membrane the nanotube is targetted. Does is create a link with the periplasm, or directly link the two cells cytoplasm. To test the two hypothesis, we tried to create a design for each of the two possibilities, with the idea in mind that if one work and the other not, we will know the answer to the question
Designs testing B. Subtilis to B. Subtilis communication
In B. Subtilis, the nanotubes is reported to connect directly the two cytoplasm. We had to find a molecule that can be expressed in the first one and trigger a genetic circuit in the second one. The principle is summed up on this image:
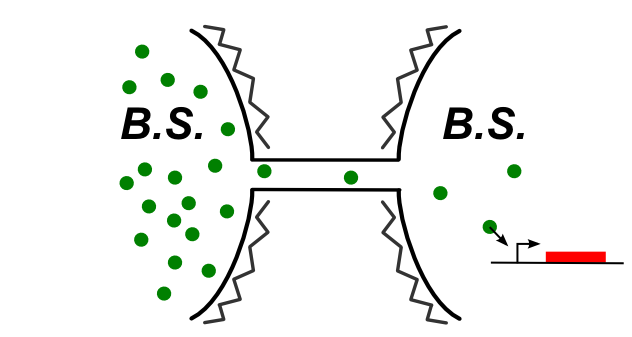
As we said earlier, the idea is to try to pass several type of molecules, with different sizes, and see if we can observe an increase in the time with the size of the molecule. The molecule choosen covers 3 order of magnitude of size. They are there classified from the biggest to the smallest.
- The T7 polymerase diffusion: The T7 RNA polymerase is the RNA polymerase of the T7 phage. This is a pretty big molecule, that recognize a very specific propoter orthogonal to the bacteria one.
- The KinA diffusion: The idea is to make a complex partner to diffuse from one cell to the other in order to trigger the sporulation of the second one, even in expodential phase
- The ComS diffusion:ComS is an inhibitor of the MecA protease in B. Subtilis. It plays a key role in the triggering of the competence mechanism. The idea is to trigger the switch of the MeKS system of the receptor cell by diffusing ComS through the nanotubes. A trick is use to prevent the first cell from sporulating.
- The amber suppressor tRNA diffusion: The principle of this design is to produce in one cell a amber supressor tRNA that will diffuse through the nanotubes. The receptor cell holds the gene for a T7 with amber stop codons that cannot be translated into a functional protein as long as the tRNA amber suppressor is not present in the cell. Once expressed, the T7amber will trigger the T7 amplification system.
Designs for B. Subtilis-E. Coli communication
As we said above, if a nanotube communication can be established between E. Coli and B. Subtilis, we don not know if the communication happens throught the cytoplasm or the periplasm. To test these two hypothesis, we have two types of designs.
If communication happens with the cytoplasm
The cheme below summ up the kind of connection that can be established if the nanotube connect the two cytoplasm. Hence, some of the design we shown above are still available from B. Subtilis to E. Coli.
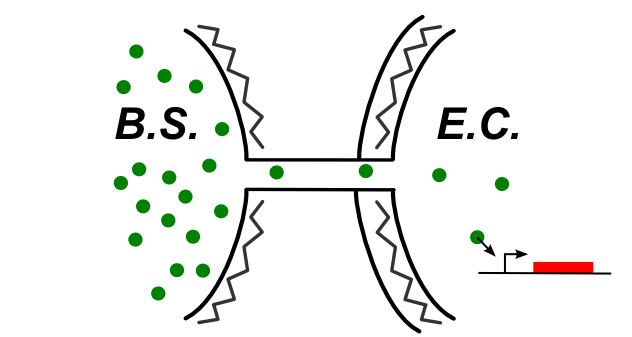
Here are the designs tested in this case:
- The T7 polymerase diffusion: The T7 RNA polymerase is the RNA polymerase of the T7 phage. This is a pretty big molecule, that recognize a very specific propoter orthogonal to the bacteria one.
- The C1(ind) diffusion: Thanks to our collaboration with the Pekin iGEM Team, we had the possibility to reuse the push-on push-off system they designed last year. The idea is to diffuse from subtilis the molecule C1 that will trigger a change in the toggle switch state
- The Xis protein diffusion: Xis is a small partner of an exisase. The latter will exise a stop codon on the DNA strand that is preventing the expression of the GFP.We had no time to go on with this design, but the idea is summed up on the linked page.
- The amber suppressor tRNA diffusion: The principle of this design is to produce in one cell a amber supressor tRNA that will diffuse through the nanotubes. The receptor cell holds the gene for a T7 with amber stop codons that cannot be translated into a functional protein as long as the tRNA amber suppressor is not present in the cell. Once expressed, the T7amber will trigger the T7 amplification system.
If communication happens with the periplasm
In case the communication happens with the periplasm, we have to think about molecules that can be then transported into the cytoplasm.

We have to think about more sophisticated approaches. The designs are as following:
- The MBP diffusion: We need a CRP+, MBP- E.Coli mutant. We produce the MBP protein in Bacillus subtilis and make it diffuse through the nanotubes. As long as the MBP has not reached the E. Coli periplasm, the cell cannot digest the maltose in the medium. The indirect induction of MalR by MBP triggers the expression of the GFP reporter.
- The OmpR diffusion: We need a OmpR- Receptor* E. Coli mutant. We produce the OmpR protein in Bacillus Subtilis. As long as the OmpR has not diffused from B. Subtilis, the signaling cascade cannot be activated. With the rescue by Bacillus Subtilis of the OmpR protein, the expression of the reporter gene is activated.
Can we conclude on these design?
If one of these designs works, we would prove that the interspecial nanotube communication exists and would find the eventual location of the connection with the membrane.
Designs for a Master-Slave system
The principle of Step 2 is to build a Master-Slave system, where the Master controls the state of the Slave cell in a monodirectional exchange.

Such a design implies the reversibility of all the sub-systems, the activators and the amplifiers in particular.
Several potential designs are summed-up below:
- The ComS system: The characterisation step of the MeKS system turn to be reversible. It can be considered as well as a Master/Slave system.
 "
"