Team:Imperial College London/Project/Chemotaxis/Testing
From 2011.igem.org

Module 1: Phyto-Route
Chemotaxis is the movement of bacteria based on attraction or repulsion of chemicals. Roots secrete a variety of compounds that E. coli are not attracted to naturally. Accordingly, we engineered a chemoreceptor into our chassis that can sense malate, a common root exudate, so that it can swim towards the root. Additionally, E. coli are actively taken up by plant roots, which will allow targeted IAA delivery into roots by our system.
Testing
Testing for chemotaxis can be split into qualitative and quantitative assays. Qualitative assays involve putting engineered E. coli and an attractant onto semi-solid agar plates and observe the movement of the microbes. If they can be observed to move towards the attractant source, they are likely to be attracted to the ligand. In quantitative assays, capillaries are filled with different concentrations of the attractant malate. Positive controls are provided by filling identical capillaries with different concentrations of serine, which E. coli naturally move towards. Negative controls are provided by filling capillaries with media that does not contain a source of attractant. The amount of bacteria that swim into each capillary is evaluated by FACS.
For simplicity, we will be working with Arabidopsis to observe the uptake of bacteria into plant roots. Arabidopsis thaliana is a common plant model organism. It belongs to the mustard family and fulfils many important requirements for model organisms. As such, its genome has been almost completely sequenced and replicates quickly, producing a large number of seeds. It is easily transformed and many different mutant strains have been constructed to study different aspects (National Institute of Health, no date). While Arabidopsis may not represent plant populations naturally occurring in arid areas threatened by desertification, it is a handy model organism we will be using to study the effect of auxin on roots, observe chemotaxis towards them and look at uptake of bacteria into the roots.
We will be using Arabidopsis to look at the uptake of our engineered bacteria into the plants. For this, we will be using wild type Arabidopsis and E. coli that constitutively express green fluorescent protein. The natural fluorescence produced by plant roots and green fluorescence produced by the bacteria can be used to image the uptake of bacteria using confocal microscopy.
1. Testing for chemotaxis towards malate
Experiments involving chemotaxis can be split to two categories, qualitative and quantitative. In the qualitative experiments, we are able to show that bacteria, which we study do or do not move towards a source, however it does not inform us at all about the cell count.
5th of August - Agar plug in assay
The simplest method for studying chemotaxis is to use agar plug in assay, which is a simple experimental method to show bacterial movement towards a localised chemical source.
Experiment is performed as follows, bacteria are added into the plate on one side, and the attractant to the other. Result is then the shape of the formed colony which grows into distorted elipse shape within the agar if the attractant is present, however colony shape remains circular if no attractant was added.
In our experiment we have used 5-α Escherichia coli cells. Cells used as positive control were transformed with pSB1C3 plasmid backbone, which conferred chloramphenicol resistance. These cells have not been modified in any other way and were used to show 5-α E. coli are capable of chemotaxis, using serine as attractant, which acts as a ligand for Tsr, an endogenous chemoreceptor. 5-α Escherichia coli competent cells were also transformed with pRK415 a working plasmid with mcpS gene and tetracycline resistance. These cells express mcpS gene from P. putida KT2440, which is chemoreceptor with malate as its ligand, and therefore malate was used as attractant for these cells during agar plug in assay. As a negative control, no attractant was added into the plate.
Amount of attractant added contributes greatly towards the result, as too little attractant added will not stimulate bacteria to move towards source, however too much attractant will result in the saturation of the medium and the bacteria will not chemotax towards source. Values (concentration of attractant, distance between colony and attractant at start etc.), necessary to perform this experiment have been worked out using modelling.
These are the values of attractant concentration we have used, when 5µl sample was added into the plate:|
|
1 |
2 |
3 |
4 |
5 |
|
Concentration |
1.884x10-3 mol/L |
1.884x10-2 mol/L |
1.884x10-1 mol/L |
0.5 mol/L |
0.75 mol/L |
|
Amount |
9.42x10-9 mol |
9.42x10-8 mol |
9.42x10-7 mol |
2.5x10-7 mol |
3.75x10-6 mol |
Note: Concentration values for 4 & 5 were not obtained from models. They were overestimations to show possible saturation of the medium with attractant.
Results:

Figure 1: Negative control pictured for the attraction E. coli cells, with added attractant malate. Rising consentrations of malate were tested, 0 M, 10-5 M, 10 -4 M, 10 -3 M, 10 -2 M, 10 -1 M. Circular colonies are observed with rising concentrations.
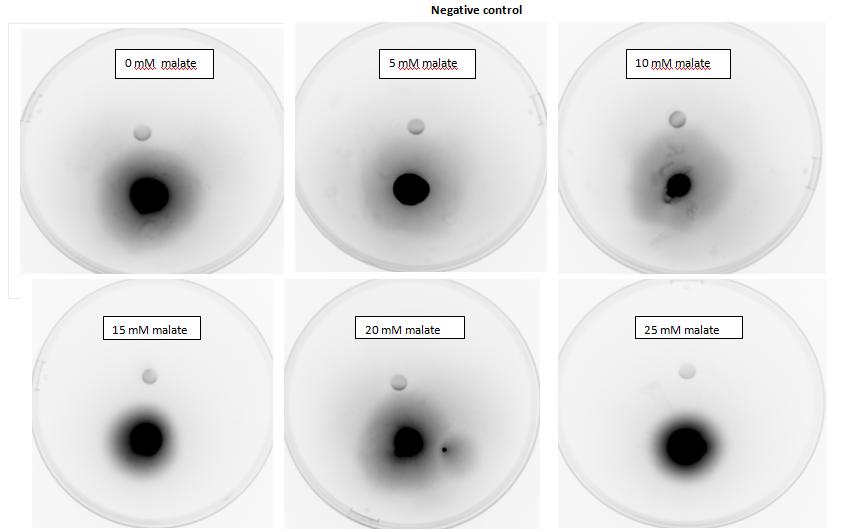
Figure 2: Negative control pictured for the attraction E. coli cells, with added attractant malate. Rising consentrations of malate in milimolar range were tested, 0 mM, 5 mM, 10 mM, 15 mM, 20 mM, 25 mM. Circular colonies are observed with rising concentrations.

Figure 3: Positive control pictured for the attraction E. coli cells, with added attractant serine, which is compatible with native E. coli chemoreceptors. Rising consentrations of serine were tested, 0 M, 10-5 M, 10 -4 M, 10 -3 M, 10 -2 M, 10 -1 M. Circular colonies are observed with 0 M & 10-5 M concentrations, result for 10 -4 M has been rendered void due to mishandling with semi - solid agar. Possible elipse shape of the 10 -3 M sample can be observed. Directed colony shape towards the attractant source is observed at 10 -2 M, and circular colony shape is observed in the 10 -1 M sample.

Figure 4: Positive control pictured for the attraction E. coli cells, with added attractant serine, which is compatible with native E. coli chemoreceptors. Rising consentrations of serine in milimolar range were tested, 0 mM, 5 mM, 10 mM, 15 mM, 20 mM, 25 mM. Circular colony can be observed at 0 mM concentration as control, at all other milimolar concentrations directed colony shape towards attractant source can be observed.
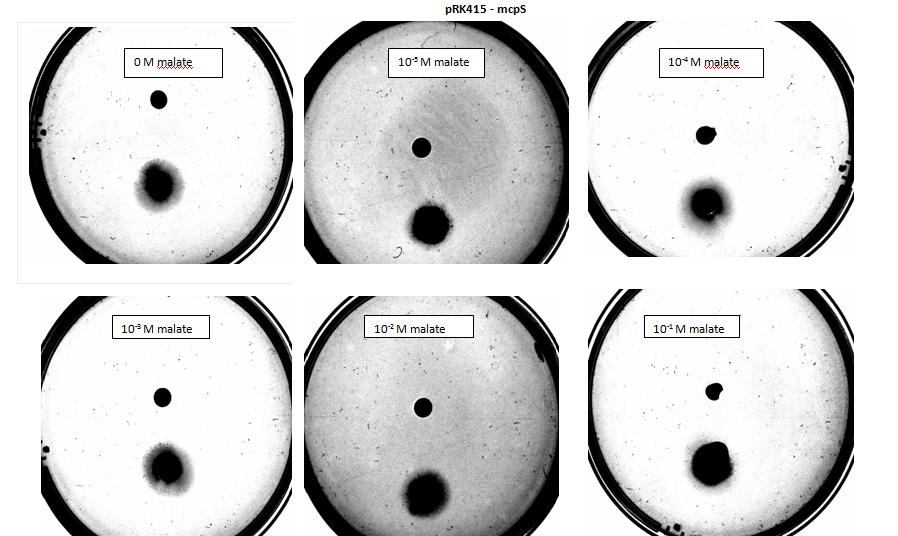
Figure 5: Modified 5α E. coli cells with mcpS on pRK415 plasmid pictured with added attractant malate. Rising consentrations of malate were tested, 0 M, 10-5 M, 10 -4 M, 10 -3 M, 10 -2 M, 10 -1 M. Circular colonies are observed with rising concentrations.

Figure 6: Modified 5α E. coli cells with mcpS on pRK415 plasmid pictured with added attractant malate. Rising consentrations of malate in milimolar range were tested, 0 mM, 5 mM, 10 mM, 15 mM, 20 mM, 25 mM. Circular colonies are observed with rising concentrations, with possible directed colony shape at 15mM and 20mM samples.

Figure 7: Modified 5α E. coli cells with PA2652 receptor on pSB1C3 plasmid pictured with added attractant malate. Rising consentrations of malate were tested, 0 M, 10-5 M, 10 -4 M, 10 -3 M, 10 -2 M, 10 -1 M. Circular colony can be observed for control, elipse shape of colonies can be observed at 10-5 & 10 -3M concentrations. Elipse shaped colony can also be observed at 10 -4 M however a large spread of bacteria can also be observed to the right side of the plate. Bacteria exposed to 10 -2 M, 10 -1 M concentrations, appear as circular colonies, however the colony spread is smaller it is possible due to saturation by malate at high concentrations.
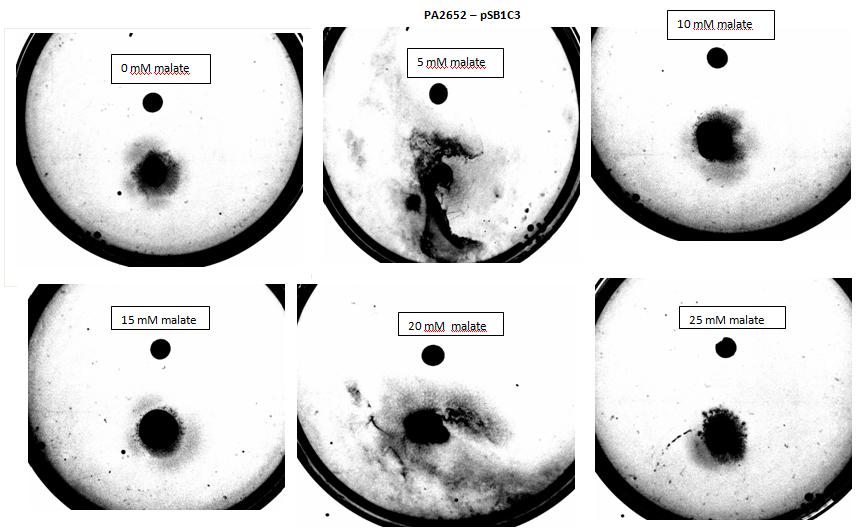
Figure 8:Modified 5α E. coli cells with PA2652 receptor on pSB1C3 plasmid pictured with added attractant malate. Rising concentrations of malate in milimolar range were tested, 0 mM, 5 mM, 10 mM, 15 mM, 20 mM, 25 mM. Circular colonies can be observed for 0 mM, 10 mM, 15 mM, 25 mM concentrations. Colonies exposed to 5 mM and 20 mM concentration of attractant were rendered void due to mishandling with semi - solid agar.
18th of August
Capillary assayquantitative, bacterial movement into a syringe/capillary based on attraction, number of bacteria measured in a capillary after 30min using FACS machine. motility medium & high bacterial OD used..
2. Tests for uptake of bacteria into roots
Wednesday, 3 August 2011
One important part of our project is uptake of our bacteria into plant roots. The observation that this occurs (albeit under controlled lab settings) is new and was only published last year. We attempted to replicate these findings.
In preparation, we met with Dr Martin Spitaler who advised us on how to prepare samples for the confocal microscopy. Confocal microscopy is much more precise than conventional light field microscopy as it eliminates background light by focusing the laser through a pinhole (Mark Scott, oral communication). The confocal microscopy will focus on imaging GFP expressing bacteria inside Arabidopsis roots to show that uptake of the bacteria takes place.
Staining of wt roots with DiD, a lipophilic dye that stains the plant membranes and does not interfere with the absorption or emission spectra of GFP and Dendra, was unsuccessful. However, natural fluorescence was measured in a root in a spectrum that does not interfere with measuring GFP. We should therefore not need to dye the roots before imaging.
Stack of wt Arabidopsis root. The root can be imaged at around 488nm. Imaging carried out by Dr Martin Spitaler.
We may also try to stain the roots with propidium iodide, which is also a strong indicator of cell wall break down.
Thursday, 4 August 2011
We prepared the GFP-expressing bacteria for plant infection. They were spun down and media was exchanged prior to incubation at 37°C to reach exponential phase. Bacteria were then spun down and resuspended in wash buffer (5mM MES) to reach OD 30. 8ml, 4ml and 2ml were added to separate flasks, containing 100ml of half-MS media each. 4ml and 6ml of wash buffer were added to the flasks containing 4ml and 2ml bacteria, respectively. 8ml of wash buffer was added to the negative control. Ten Arabidopsis seedlings were distributed into each of the flasks. Incubation was carried out for 15 hours prior to imaging.
Friday, 5 August 2011
Prior to imaging, roots were washed in PBS to wash off bacteria and facilitate imaging. We imaged the plants incubated with 8ml of bacteria and were able to find bacteria inside one of the roots. A 3D picture was taken of uninfected roots and roots containing bacteria by taking a Z stackk image using confocal microscopy.
This video shows an image put together from successive images taken at different depth levels. The GFP-expressing bacteria are clearly visible within the root.
We also imaged roots that did not contain bacteria via a similar Z-stack scan. This image is shown below.
We repeated this experiment at a later date with plants that had been allowed to grow for a longer period of time. The bacteria were predominantly found in root hairs and inside of cells on the root surface.

3. Tracking of cell viability using Dendra
Dendra-expressing bacteria were also taken up into plant roots. Using a confocal microscope, we photoconverted the Dendra protein from green to red fluorescence. Conversion with the 405nm laser was completed after about 15 rounds of bleaching at 50% laser intensity with the pinhole set to 3 airy units.

Dendra photoconversion in bacteria taken up inside plant roots. 1 is the area photoconverted using the 405nm laser. 2 is an individual bacterium whose Dendra protein has undergone photoconversion. 3 is a negative control consisting of a non-photoconverted bacterium. The bacteria found inside the roots can be seen on the right. The data on the left displays the conversion from green to red fluorescence for the highlighted areas. Ch2: emission in green spectrum. Ch3: emission in red spectrum. ChD: brightfield emission.
 "
"




