Team:Edinburgh/Cell Display
From 2011.igem.org
| (40 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
<html><script type="text/javascript" >$(document).ready(function() { | <html><script type="text/javascript" >$(document).ready(function() { | ||
getMenus('home', 'home_cell'); | getMenus('home', 'home_cell'); | ||
| - | |||
}); </script></html> | }); </script></html> | ||
<div class="main_body"> | <div class="main_body"> | ||
| - | + | <p class="h1">Cell Surface Display: Proposals</p> | |
| - | + | The first proposed system our feasibility study will examine, while searching for a way to keep extracellular enzymes close together, is based on displaying proteins at high density on an <span class="hardword" id="ec">''E. coli''</span> outer membrane. This type of display is called "cell surface display". | |
| + | |||
| + | We will attempt to design such a system for <span class="hardword" id="cellulase">cellulases</span>, and see if we can get it to work. | ||
==Outline== | ==Outline== | ||
| - | + | In order to get a normal enzyme displayed on the ''E. coli'' outer membrane, the enzyme must be fused to a carrier protein; that is, one which is naturally transported to the outer membrane. | |
| - | + | [https://2009.igem.org/Team:Berkeley_Wetlab/Assay_Protocols Berkeley 2009] tried several different carrier proteins with several different passenger enzymes, and had success in many areas. However, when they tried attaching cellulases, they [https://2009.igem.org/Team:Berkeley_Wetlab/Passenger:_Cellulases weren't so successful] - of the two quantified cellulases, one worked just as well without the carrier (Cel5b) and the other didn't work (Cel9a, as compared to negative control). | |
| + | We will try a different carrier. The <span class="hardword" id="biobrick">BioBrick</span> [http://partsregistry.org/Part:BBa_K265008 BBa_K265008] made by [https://2009.igem.org/Team:UC_Davis UC Davis 2009] is a synthetic, codon-optimised sequence, based on [http://www.ncbi.nlm.nih.gov/nuccore/AF013159 GenBank AF013159] and coding for the first 211 and last 97 amino acids of <span class="hardword" id="inp">Ice Nucleation Protein</span> (INP, normally coded by the ''inaK'' gene) from the bacterium <span class="hardword" id="ps">Pseudomonas syringae</span>. It seems promising as a carrier of enzymes. Fusions are carried out at the INP C terminal. | ||
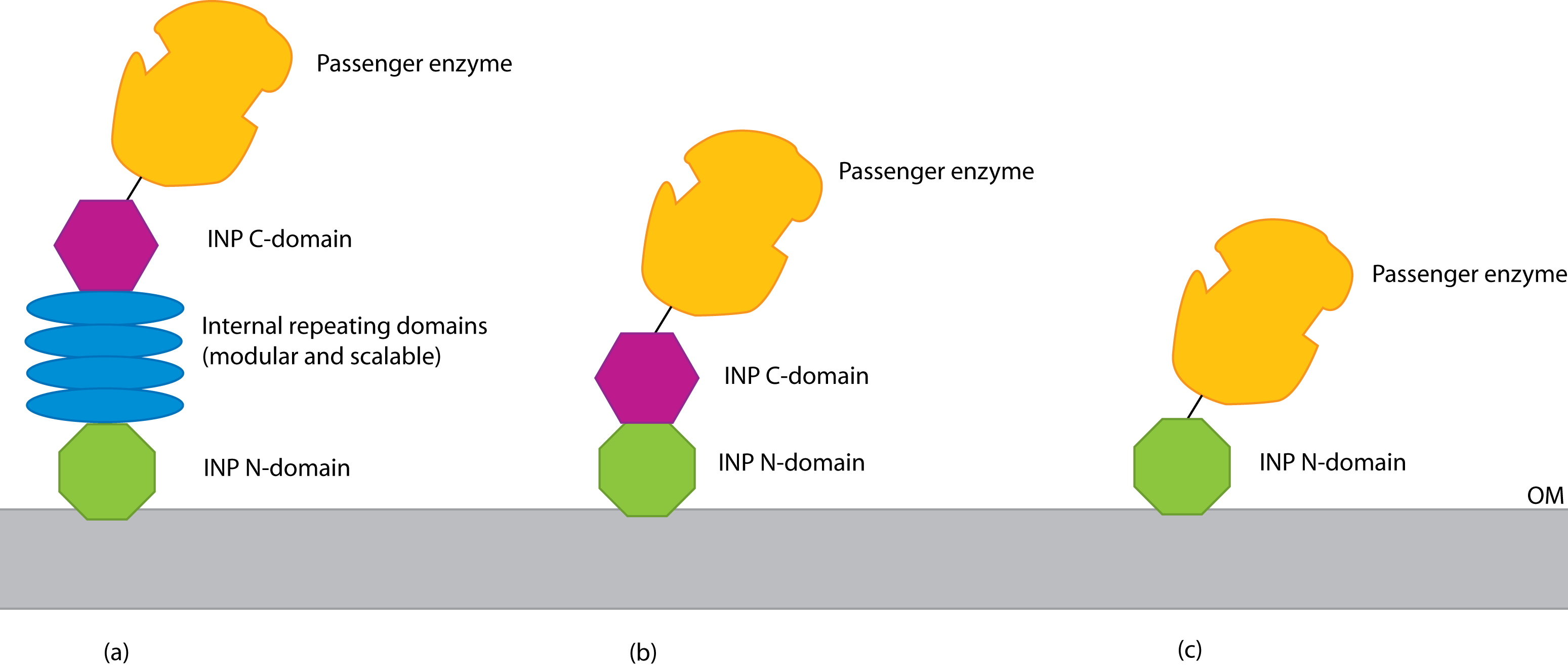
[[File:Three-displays.png|thumb|center|600px|Three strategies for INP-based cell display. After [http://www.sciencedirect.com/science/article/pii/S016777991000199X Van Bloois ''et al'' (2011)]]] | [[File:Three-displays.png|thumb|center|600px|Three strategies for INP-based cell display. After [http://www.sciencedirect.com/science/article/pii/S016777991000199X Van Bloois ''et al'' (2011)]]] | ||
| + | [http://www.sciencedirect.com/science/article/pii/S016777991000199X Van Bloois ''et al'' (2011)] speak highly of INP, and claim that it can be displayed at a copy number of around 100,000 copies per cell without affecting viability. | ||
INP has major domains at its N and C terminals, as well as a number of internal repeating domains. There seem to be three strategies for using INP (see figure): | INP has major domains at its N and C terminals, as well as a number of internal repeating domains. There seem to be three strategies for using INP (see figure): | ||
| - | * Use the entire INP protein; fuse at | + | * Use the entire INP protein; fuse at its C terminal |
| - | * Delete the INP internal domains; fuse at | + | * Delete the INP internal domains; fuse at its C terminal |
* Delete all of INP except the N domain; fuse at the new C terminal | * Delete all of INP except the N domain; fuse at the new C terminal | ||
| - | + | [http://partsregistry.org/Part:BBa_K265008 BBa_K265008] should be suitable for the 2nd strategy. | |
===Linkers=== | ===Linkers=== | ||
| - | It | + | It is probably desirable to create <span class="hardword" id="linker">linkers</span> between the carrier and the protein of interest, to give the proteins space to fold. The new assembly protocol that we are investigating — [[Team:Edinburgh/BioSandwich|BioSandwich]] — should be ideal for this. |
| - | === | + | ===Complete system=== |
| - | + | The complete 3 cellulase system could contain a promoter, driving expression of three coding fusions: | |
| - | * | + | * INP -- (Linker) -- endoglucanase (e.g. [http://partsregistry.org/Part:BBa_K392006 BBa_K392006]) |
| - | * | + | * INP -- (Linker) -- exoglucanase (e.g. [http://partsregistry.org/Part:BBa_K392007 BBa_K392007]) |
| - | * | + | * INP -- (Linker) -- β-glucosidase (e.g. [http://partsregistry.org/Part:BBa_K392008 BBa_K392008]) |
===An alternative: protein chains=== | ===An alternative: protein chains=== | ||
| Line 44: | Line 47: | ||
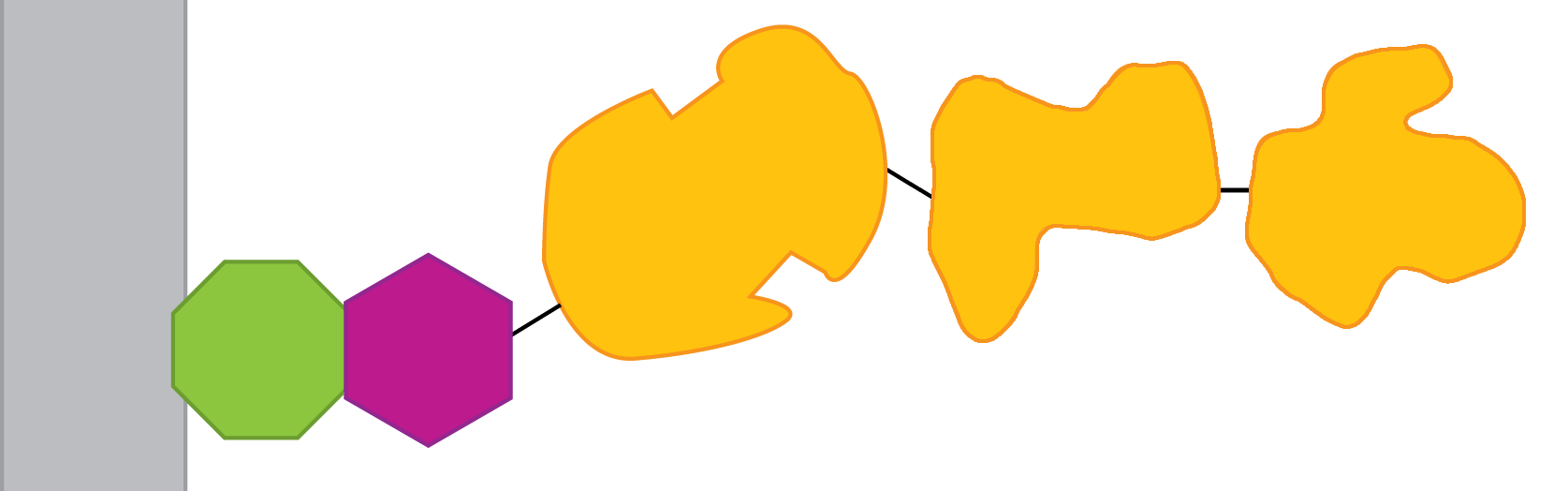
[[File:INP-chain.png|thumb|center|600px|The protein chain idea: a long fusion protein is created with INP fused to (say) 3 enzymes in a row...]] | [[File:INP-chain.png|thumb|center|600px|The protein chain idea: a long fusion protein is created with INP fused to (say) 3 enzymes in a row...]] | ||
| - | Instead of making three different fusions, it might be possible to make one fusion that had all three cellulase enzymes linked together: | + | Instead of making three different fusions, it might be possible to make one fusion that had all three cellulase enzymes linked together; we call this "beads on a string". As it happens, the exoglucanase (Cex) and the endoglucanase (CenA) both have a cellulose-binding module (CBM), but they are at different ends of the sequence. So here's the plan: |
| - | * | + | * Create a fusion of: |
| - | + | ** '''Exoglucanase''' (catalytic domain) -- '''CBM''' -- '''Endoglucanase''' (catalytic domain) | |
| - | + | ** This can be done by homology or by introducing an NcoI site into both Exo- and Endoglucanase at the appropriate locations, then ligating and doing fusion PCR. | |
| - | + | * We can then use KpnI in a similar way to attach a '''β-glucosidase''' at either end. | |
| - | + | * Then attach INP. | |
==Genetic instability== | ==Genetic instability== | ||
| Line 58: | Line 61: | ||
This will not be a problem in the JM109 lab strain, which lacks an important <span class="hardword" id="recombination">recombination</span> enzyme. As for the use of this technology in industry, it will be possible to overcome this problem simply by synthesising coding sequences with as many altered (but <span class="hardword" id="synonymouscodon">synonymous</span>) codons as possible. We have written a software tool for designing such sequences... see the [[Team:Edinburgh/Genetic instability|genetic instability]] page. | This will not be a problem in the JM109 lab strain, which lacks an important <span class="hardword" id="recombination">recombination</span> enzyme. As for the use of this technology in industry, it will be possible to overcome this problem simply by synthesising coding sequences with as many altered (but <span class="hardword" id="synonymouscodon">synonymous</span>) codons as possible. We have written a software tool for designing such sequences... see the [[Team:Edinburgh/Genetic instability|genetic instability]] page. | ||
| - | == | + | ==Proof of concept: YFP== |
| - | + | As far as we know, nobody has used [http://partsregistry.org/Part:BBa_K265008 BBa_K265008] for cell display. We could prove that it works by simply displaying the Yellow Fluorescent Protein on INP. Indeed, something similar was achieved by [http://www.postech.edu/~hjcha/INP-N-GFP-OPH.pdf Li ''et al'' (2004)] and [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2009.01724.x/abstract Li ''et al'' (2009)] for a different version of the gene. | |
| - | + | ==Results== | |
| + | |||
| + | Please see the team's [[Team:Edinburgh/Data | Data Page]] for information about how far we got with this project. | ||
==References== | ==References== | ||
| + | |||
| + | * Li L, Kang DG, Cha HJ (2004) [http://www.postech.edu/~hjcha/INP-N-GFP-OPH.pdf Functional display of foreign protein on surface of ''Escherichia coli'' using N-terminal domain of Ice Nucleation Protein]. ''Biotechnology and Bioengineering'' '''85'''(2): 214-221 (doi: 10.1002/bit.10892). | ||
| + | |||
| + | * Li Q, Yu Z, Shao X, He J, Li L (2009) [http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2009.01724.x/abstract Improved phosphate biosorption by bacterial surface display of phosphate-binding protein utilizing ice nucleation protein]. ''FEMS Microbiology Letters'' '''299'''(1): 44-52 (doi: 10.1111/j.1574-6968.2009.01724.x). | ||
* Van Bloois E, Winter RT, Kolmar H, Fraaije MW (2011) [http://www.sciencedirect.com/science/article/pii/S016777991000199X Decorating microbes: surface display of proteins on ''Escherichia coli'']. ''Trends in Biotechnology'' '''29'''(2): 79-86 (doi: 10.1016/j.tibtech.2010.11.003). | * Van Bloois E, Winter RT, Kolmar H, Fraaije MW (2011) [http://www.sciencedirect.com/science/article/pii/S016777991000199X Decorating microbes: surface display of proteins on ''Escherichia coli'']. ''Trends in Biotechnology'' '''29'''(2): 79-86 (doi: 10.1016/j.tibtech.2010.11.003). | ||
| - | + | ||
</div> <!-- /main_body--> | </div> <!-- /main_body--> | ||
<html></div> <!-- /mids --></html> | <html></div> <!-- /mids --></html> | ||
Latest revision as of 20:01, 28 October 2011
Cell Surface Display: Proposals
The first proposed system our feasibility study will examine, while searching for a way to keep extracellular enzymes close together, is based on displaying proteins at high density on an E. coli outer membrane. This type of display is called "cell surface display".
We will attempt to design such a system for cellulases, and see if we can get it to work.
Contents |
Outline
In order to get a normal enzyme displayed on the E. coli outer membrane, the enzyme must be fused to a carrier protein; that is, one which is naturally transported to the outer membrane.
Berkeley 2009 tried several different carrier proteins with several different passenger enzymes, and had success in many areas. However, when they tried attaching cellulases, they weren't so successful - of the two quantified cellulases, one worked just as well without the carrier (Cel5b) and the other didn't work (Cel9a, as compared to negative control).
We will try a different carrier. The BioBrick BBa_K265008 made by UC Davis 2009 is a synthetic, codon-optimised sequence, based on GenBank AF013159 and coding for the first 211 and last 97 amino acids of Ice Nucleation Protein (INP, normally coded by the inaK gene) from the bacterium Pseudomonas syringae. It seems promising as a carrier of enzymes. Fusions are carried out at the INP C terminal.
Van Bloois et al (2011) speak highly of INP, and claim that it can be displayed at a copy number of around 100,000 copies per cell without affecting viability.
INP has major domains at its N and C terminals, as well as a number of internal repeating domains. There seem to be three strategies for using INP (see figure):
- Use the entire INP protein; fuse at its C terminal
- Delete the INP internal domains; fuse at its C terminal
- Delete all of INP except the N domain; fuse at the new C terminal
BBa_K265008 should be suitable for the 2nd strategy.
Linkers
It is probably desirable to create linkers between the carrier and the protein of interest, to give the proteins space to fold. The new assembly protocol that we are investigating — BioSandwich — should be ideal for this.
Complete system
The complete 3 cellulase system could contain a promoter, driving expression of three coding fusions:
- INP -- (Linker) -- endoglucanase (e.g. BBa_K392006)
- INP -- (Linker) -- exoglucanase (e.g. BBa_K392007)
- INP -- (Linker) -- β-glucosidase (e.g. BBa_K392008)
An alternative: protein chains
Instead of making three different fusions, it might be possible to make one fusion that had all three cellulase enzymes linked together; we call this "beads on a string". As it happens, the exoglucanase (Cex) and the endoglucanase (CenA) both have a cellulose-binding module (CBM), but they are at different ends of the sequence. So here's the plan:
- Create a fusion of:
- Exoglucanase (catalytic domain) -- CBM -- Endoglucanase (catalytic domain)
- This can be done by homology or by introducing an NcoI site into both Exo- and Endoglucanase at the appropriate locations, then ligating and doing fusion PCR.
- We can then use KpnI in a similar way to attach a β-glucosidase at either end.
- Then attach INP.
Genetic instability
In order to display several different proteins on one bacterium using the first strategy, it will be necessary to have several copies of the INP gene fused to different enzymes. The presence of repeated sequences on a plasmid can lead to genetic instability.
This will not be a problem in the JM109 lab strain, which lacks an important recombination enzyme. As for the use of this technology in industry, it will be possible to overcome this problem simply by synthesising coding sequences with as many altered (but synonymous) codons as possible. We have written a software tool for designing such sequences... see the genetic instability page.
Proof of concept: YFP
As far as we know, nobody has used BBa_K265008 for cell display. We could prove that it works by simply displaying the Yellow Fluorescent Protein on INP. Indeed, something similar was achieved by Li et al (2004) and Li et al (2009) for a different version of the gene.
Results
Please see the team's Data Page for information about how far we got with this project.
References
- Li L, Kang DG, Cha HJ (2004) Functional display of foreign protein on surface of Escherichia coli using N-terminal domain of Ice Nucleation Protein. Biotechnology and Bioengineering 85(2): 214-221 (doi: 10.1002/bit.10892).
- Li Q, Yu Z, Shao X, He J, Li L (2009) Improved phosphate biosorption by bacterial surface display of phosphate-binding protein utilizing ice nucleation protein. FEMS Microbiology Letters 299(1): 44-52 (doi: 10.1111/j.1574-6968.2009.01724.x).
- Van Bloois E, Winter RT, Kolmar H, Fraaije MW (2011) Decorating microbes: surface display of proteins on Escherichia coli. Trends in Biotechnology 29(2): 79-86 (doi: 10.1016/j.tibtech.2010.11.003).
 "
"