Team:EPF-Lausanne/Our Project/T7 promoter variants/t7make/characterization/iptg
From 2011.igem.org
Characterization using IPTG Induction
LacI repression
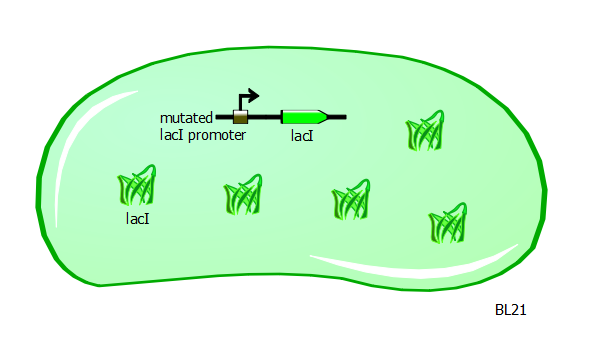
With T7 and T7-lac promoter variants in hand, we want to characterize their relative strengths. To obtain the right set-up, we transform the promoter variant plasmids into a different strain of E. coli called BL21. These cells have a mutation in the promoter for the lacI gene. As a result of this mutation, LacI protein is overproduced and is found abundantly in these cells.
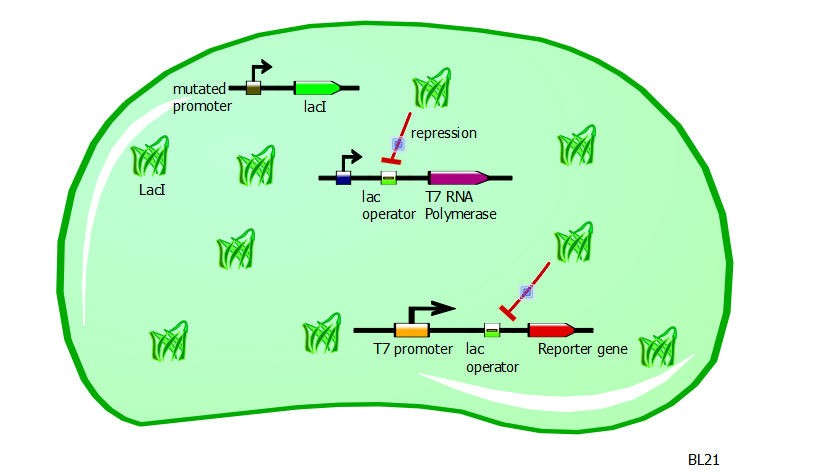
In this same strain, the gene for the T7 RNA polymerase is preceded by a lac operator. Since LacI is a repressor and is strongly present in BL21 cells, the production of T7 RNA polymerases is severely repressed.
With very few T7 RNA polymerases available, there is very little recognition and binding of T7 promoters and consequently very little expression of the gene driven by the T7 promoter. Moreover, the expression of a gene driven by a T7-lac promoter would be even less, since the presence of LacI would block any action of the T7 RNA polymerase.
IPTG induction
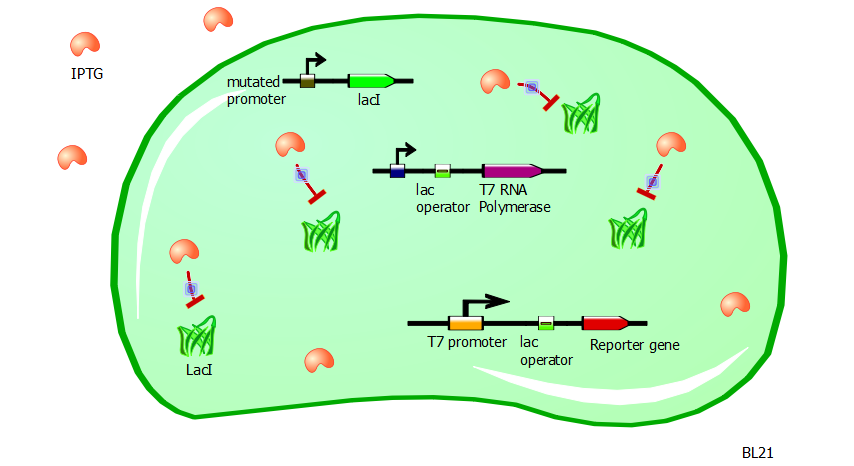
With such a system in place, the transformation of our T7 and T7-lac promoter variant plasmids into BL21 cells would produce little to no reporter expression. Now to induce promoter activity, we rely on the fact that the chemical IPTG blocks the repressor action of LacI.
Without LacI inhibition, the cell can resume production of T7 RNA polymerases. These in turn can bind to the T7 and T7-lac promoters to express the reporter gene without interruption. So the adding of IPTG to a BL21 cell culture containing these T7 and T7-lac promoter variant plasmids ought to produce high levels of RFP or Lysis expression. Since lysis is a rather binary process (either the cell is lysed or it is not), we use RFP fluorescence as a gauge of promoter strength and efficiency.
 "
"