Team:Harvard/Notebook
From 2011.igem.org
| Sun | Mon | Tue | Wed | Thu | Fri | Sat |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| 19 | 20 | 21 | 22 | 23 | 24 | 25 |
| 26 | 27 | 28 | 29 | 30 |
| Sun | Mon | Tue | Wed | Thu | Fri | Sat | |
|---|---|---|---|---|---|---|---|
| 1 | 2 | ||||||
| 3 | 4 | 5 | 6 | 7 | 8 | ||
| 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 17 | 18 | 19 | 20 | 21 | 22 | 23 | |
| 24 | 25 | 26 | 27 | 28 | 29 | 30 | |
| 31 |
| Sun | Mon | Tue | Wed | Thu | Fri | Sat |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 21 | 22 | 23 | 25 | 25 | 26 | 27 |
| 28 | 29 | 30 | 31 |
June 6th
First day of iGEM!June 7th
Miniprep of pKD42 (lambda red)
The lambda red plasmid is needed to enable the recombination used to insert the selection/expression systems into our E. coli cultures.
Procedure: followed Qiagen Kit instructions, each student (8) using 1 mL cell suspension
Results: DNA reasonably pure (260/280 between 1.8 and 2) and between 25 and 50 ng/µLJune 8th
PCR to connect ultramers into OZ052 (Zif268 F2 triplicate, GCCGATGTC)and OZ123 (Zif268 F2 triplicate, GAGTGGTTA):
OZ052:
- 3µL OZ052_F (10µM stock)
- 3µL OZ052_R (10µM stock)
- 5µL 10x Pfx amplification buffer
- 1.5µL dNTPs
- 1µL MgSO4
- 0.4µL DNA polymerase
- 36.1µL ddH2O
OZ123:
- 3µL OZ123_F (10µM stock)
- 3µL OZ123_R (10µM stock)
- 5µL 10x Pfx amplification buffer
- 1.5µL dNTPs
- 1µL MgSO4
- 0.4µL DNA polymerase
- 36.1µL ddH2O
Parameters:
- 1) 94⁰C for 5 min
- 2) 94⁰C for 15 sec
- 3) 60⁰C for 30 sec
- 4) 68⁰C for 1 min
- 5) Repeat 2-4 for 25 cycles
- 6) 68⁰C for 5 min
- 7) 4⁰C forever
June 9th - Wet Lab
- Created cell culture with selection construct (contains ZFB, His3, pyrF on plasmid) and reporter RFP (this will be used to test positive control ZFs, cells fluoresce green when ZF binds)
- Picked colonies, grew in LB/amp liquid media until mid-log
- 3 mL of LB, 1.5 µL of 2000x amp
- Once mid-log reached, created glycerol stock, stored stock at -80⁰C.
300 µL bacteria, 1200 µL 80% glycerolThis should have been 1200 µL bacteria media, 300 µL 80% glycerol (Corrected 6/14/2011) (80% pure glycerol, 20% molecular grade water)
- Spiked new tubes of media with 25 µL bacteria from the mid-log tube to leave overnight
- Picked colonies, grew in LB/amp liquid media until mid-log
NOTE: reporter RFP did not grow to mid-log by end of day, will let grow overnight to saturation and continue creating glycerol stock tomorrow.
- Plated selection strain from gel stab onto tet plate.
- Began primer design for creating the kan/selection construct fusion.
June 9th - Bioinformatics
Today we focused on reacquainting and familiarizing ourselves with Python. We completed the parsing (reading in) of the sequence and amino acid data so that it is easy to work with: by substituting each amino acid abbreviation (ex. A, N) with its numeric equivalent (ex. 1, 14), we can use a lot of nice math comparisons instead of messy letter/"string" comparisons.
After that, we worked on counting the number of times each amino acid appears in each of the 7 positions (unfortunately given by -1,1,2,3,5,6,7), and counting the number of times amino acids are next to each other (ex. ACTQRNF has AC, CT, TQ, etc pairings). Taken overall, we found that L is overwhelmingly in position 5.
| Acid | -1 | 1 | 2 | 3 | 5 | 6 | 7 |
| A | 77 | 140 | 210 | 197 | 0 | 312 | 85 |
| C | 12 | 24 | 1 | 6 | 14 | 0 | 0 |
| D | 413 | 16 | 694 | 258 | 0 | 142 | 14 |
| E | 125 | 74 | 152 | 107 | 0 | 58 | 132 |
| F | 0 | 0 | 22 | 0 | 10 | 0 | 0 |
| G | 12 | 201 | 328 | 125 | 0 | 177 | 62 |
| H | 93 | 144 | 232 | 652 | 0 | 51 | 17 |
| I | 70 | 21 | 3 | 26 | 0 | 94 | 73 |
| K | 108 | 372 | 46 | 169 | 6 | 321 | 52 |
| L | 176 | 37 | 20 | 22 | 3325 | 75 | 55 |
| M | 36 | 54 | 5 | 28 | 0 | 31 | 10 |
| N | 23 | 150 | 129 | 940 | 0 | 182 | 61 |
| P | 3 | 298 | 77 | 7 | 0 | 36 | 8 |
| Q | 813 | 158 | 180 | 13 | 0 | 136 | 30 |
| R | 870 | 539 | 137 | 55 | 3 | 428 | 2517 |
| S | 99 | 970 | 859 | 278 | 0 | 140 | 12 |
| T | 243 | 134 | 223 | 350 | 0 | 834 | 83 |
| V | 166 | 26 | 27 | 115 | 0 | 341 | 146 |
| W | 19 | 0 | 13 | 0 | 0 | 0 | 0 |
| Y | 0 | 0 | 0 | 10 | 0 | 0 | 1 |
For pairings, we found patterns, but none as obvious as the L-in-position-5. Read this like a multiplication table: the intersection of L row and M column is how often that pairing was observed.
| ' | A | C | D | E | F | G | H | I | K | L | M | N | P | Q | R | S | T | V | W | Y |
| A | 10 | 0 | 99 | 55 | 0 | 29 | 122 | 20 | 32 | 332 | 2 | 59 | 55 | 63 | 255 | 87 | 24 | 43 | 0 | 0 |
| C | 0 | 0 | 15 | 0 | 0 | 3 | 0 | 0 | 0 | 5 | 0 | 0 | 6 | 0 | 31 | 6 | 14 | 0 | 0 | 0 |
| D | 99 | 15 | 94 | 92 | 0 | 39 | 62 | 6 | 84 | 342 | 15 | 120 | 55 | 42 | 277 | 290 | 87 | 21 | 0 | 8 |
| E | 55 | 0 | 92 | 42 | 0 | 34 | 77 | 1 | 38 | 141 | 2 | 39 | 4 | 29 | 134 | 28 | 90 | 26 | 0 | 1 |
| F | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 22 | 4 | 0 | 2 | 4 | 6 | 0 | 0 | 0 |
| G | 29 | 3 | 39 | 34 | 0 | 38 | 56 | 0 | 14 | 126 | 1 | 95 | 28 | 47 | 119 | 125 | 38 | 7 | 0 | 0 |
| H | 122 | 0 | 62 | 77 | 0 | 56 | 118 | 9 | 103 | 498 | 4 | 88 | 24 | 26 | 87 | 159 | 70 | 2 | 0 | 0 |
| I | 20 | 0 | 6 | 1 | 10 | 0 | 9 | 6 | 8 | 95 | 3 | 5 | 17 | 3 | 62 | 16 | 17 | 4 | 0 | 0 |
| K | 32 | 0 | 84 | 38 | 0 | 14 | 103 | 8 | 84 | 386 | 24 | 44 | 19 | 102 | 269 | 163 | 113 | 22 | 1 | 0 |
| L | 332 | 5 | 342 | 141 | 0 | 126 | 498 | 95 | 386 | 174 | 32 | 686 | 16 | 112 | 362 | 276 | 875 | 360 | 0 | 8 |
| M | 2 | 0 | 15 | 2 | 0 | 1 | 4 | 3 | 24 | 32 | 0 | 7 | 2 | 11 | 39 | 14 | 3 | 1 | 0 | 0 |
| N | 59 | 0 | 120 | 39 | 22 | 95 | 88 | 5 | 44 | 686 | 7 | 8 | 36 | 28 | 120 | 254 | 84 | 34 | 1 | 0 |
| P | 55 | 6 | 55 | 4 | 4 | 28 | 24 | 17 | 19 | 16 | 2 | 36 | 0 | 3 | 29 | 150 | 21 | 13 | 11 | 0 |
| Q | 63 | 0 | 42 | 29 | 0 | 47 | 26 | 3 | 102 | 112 | 11 | 28 | 3 | 100 | 261 | 314 | 125 | 19 | 0 | 0 |
| R | 255 | 31 | 277 | 134 | 2 | 119 | 87 | 62 | 269 | 362 | 39 | 120 | 29 | 261 | 618 | 343 | 504 | 281 | 0 | 0 |
| S | 87 | 6 | 290 | 28 | 4 | 125 | 159 | 16 | 163 | 276 | 14 | 254 | 150 | 314 | 343 | 592 | 173 | 91 | 0 | 0 |
| T | 24 | 14 | 87 | 90 | 6 | 38 | 70 | 17 | 113 | 875 | 3 | 84 | 21 | 125 | 504 | 173 | 154 | 28 | 0 | 0 |
| V | 43 | 0 | 21 | 26 | 0 | 7 | 2 | 4 | 22 | 360 | 1 | 34 | 13 | 19 | 281 | 91 | 28 | 12 | 0 | 0 |
| W | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Y | 0 | 0 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
June 10th - Wet Lab
- What we learned today: don't put E. coli plates in the -20C freezer!
- Observed a well populated selection strain plate and placed it in the 4C refrigerator
- Took the selection construct culture and extracted the plasmid through miniprep
- Observed 260/280 ratio of 1.90 and 1.88 through Nanodrop
- Observed concentrations of 87.7 and 100.6 ng/µL through Nanodrop
- Made 10 new agar plates with LB and amp
June 10th - Bioinformatics
Visualizations
We spent the first few hours today making cool visualizations and graphs of the data we found on the 9th: heatmaps turned out to be an annoying limitation of Python, so a Python/R hybrid was used, and bar charts were made exclusively in Python. See the dropbox for our pretty (and hopefully informative compared to spreadsheets) charts/graphs.
We then started work on TNN and GNN properties specifically (essentially repeating the June 9th work, but confined to smaller data sets). There are some differences between TNN and GNN: see graphs in dropbox. We decided that there was not enough data for fingers that bind to ANN and CNN triplets to perform significant analysis on it.
- Overall, similar color clusters are found in the heatmaps. In all cases, L and N are often placed consecutively on the helix. There are fewer clusters of high frequency when looking at TNN binders.
We then, using the theorized framework from a paper (2011 Persikov [http://iopscience.iop.org.ezp-prod1.hul.harvard.edu/1478-3975/8/3/035010/]), tried to match amino acid binding to each base pair to see if there was a pattern. See dropbox document .../bioinformatics/Binding Frequency for that data. There's a lot of it.
Properties of amino acids
We then worked on finding properties of the each position (hydrophobic/phillic, non/polar):
Hydrophilic vs Hydrophobic
| Position | Very Phobic | Hydrophobic | Neutral | Hydrophillic |
| 6 | 285 | 85 | 204 | 2782 |
| 5 | 542 | 312 | 1334 | 1169 |
| 4 | 3334 | 14 | 0 | 9 |
| 3 | 191 | 203 | 1417 | 1536 |
| 2 | 91 | 211 | 1819 | 1236 |
| 1 | 138 | 164 | 1604 | 1451 |
| -1 | 468 | 90 | 1257 | 1542 |
Polar vs Nonpolar
| Position | Polar | Nonpolar |
| 6 | 2917 | 440 |
| 5 | 2290 | 1067 |
| 4 | 9 | 3348 |
| 3 | 2830 | 527 |
| 2 | 2652 | 705 |
| 1 | 2555 | 802 |
| -1 | 2784 | 573 |
June 13th - Wet Lab
The control zinc fingers OZ052 and OZ123 were amplified with overhanging primers to allow its insertion into the Wolfe plasmid:
Overhang PCR for ultramers: the template was the product of the ultramer PCR (see 6/8/11), and several concentrations were used
In all the tubes:
- 5 µL Pfx amplification buffer
- 1.5 µL dNTPs
- 1 µL MgSO4
- 0.4 µL polymerase
- 38.1 µL ddH2O
- 1.5 µL OZ052_up and 1.5 µL OZ052_down OR 1.5 µL OZ123_up and 1.5 µL OZ123_down
In OZ052 (1) and OZ123 (1):
- 1 µL of ultramer PCR product
In OZ052 (1:10) and OZ123 (1:10):
- 1 µL of a 1 in 10 dilution of ultramer PCR product
In OZ052 (1:100) and OZ123 (1:100):
- 1 µL of a 1 in 100 dilution of ultramer PCR product
Parameters:
- 94⁰C for 5 min
- 94⁰C for 15 sec
- 55⁰C for 30 sec
- 68⁰C for 30 sec
- Repeat steps 2-4 for 25 cycles
- 68⁰C for 5 min
- 4⁰C forever
Gel to verify proper amplification (1% agarose gel, 10 µL 1 kb ladder, 120 V):
The OZ052 lanes (1-3) had bands at the proper length (328 bp) at all three concentrations, although there were several fainter bands likely from side products. Only the undiluted OZ123 lane showed any bands, and from the faint band at 328 and the stronger band around 250 it appears that the PCR did not work well, and the majority of the product was the ultramer from the first PCR.
PCR around vector: the template used was the Wolfe selection construct plasmid miniprepped 6/10/11 (100.6 ng/µL stock)
Reagents the same as above except:
- 1.5 µL of Wolfe_F and 1.5 µL of Wolfe_R primers to each tube
- plasmid tube (1 ng) given 1 ng of template (1 µL of a 1 in 100 dilution)
- plasmid tube (10 ng) given 10 ng of template (1 µL of a 1 in 10 dilution)
Parameters same as above except:
- elongation (step 4) 5 minutes (vector approximately 5 kb)
Gel to verify proper amplification (1% agarose, 10 µL 1 kb ladder, 170V)
There were no bands of the correct size in the lanes. The only band that appeared was a faint, short band in one lane that likely was a primer. Since the DNA ladder worked, the problem likely was not with the electrophoresis but with the PCR reaction, perhaps due to issues with the primers.
Gel images
June 13th - Bioinformatics
Today we started work on a program to statistically generate possible sequences.
The four functions needed to do this are:
- generate(matrix, pseudocounts (lambda), dependency tuples)
- takes a matrix of zinc-finger AA position counts, a list of dependent amino acid pairs, and a pseudocount multiplier and generates a list of potential amino acid sequences weighted by independent and dependent probabilities
- add_pseudo(dependent matrix row,independent matrix row)
- given a matrix row of dependent counts (i.e. how many times 'a' occurs at position n when 'b' is set to some AA at position m) and a row of independent matrix counts (how many times 'a' occurs at n regardless of b's AA) return an adjusted matrix row, based on the dependent matrix row, that has pseudocounts added to the values that are empty in the dependent matrix row but filled in the independent matrix row.
- generate_indep(matrix)
- randomly pick an amino acid, given a matrix row, from a weighted random distribution based on the values in the row
- generate_dep(indep_row, dep_row, lambda)
- add pseudo counts (call add_pseudo) and generate a dependent random call for a position (using generate_indep on the adjusted matrix)
July 1st
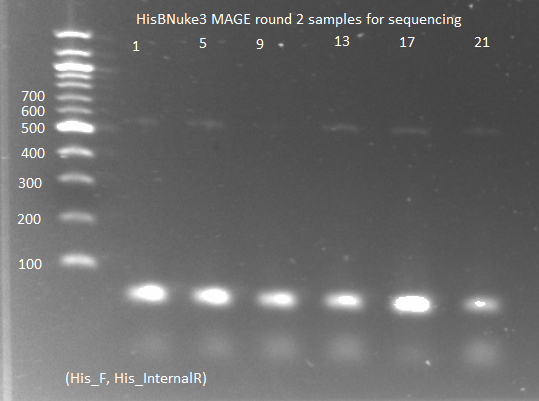
HisBNuke3 MAGE results:
- All the MAGE round 1 colonies grew overnight in the LB but not the NM medium: either they all had the HisB gene knocked out (unlikely) or there is something wrong with the NM medium. As a control we grew (∆HisB)∆pyrF∆rpoZ+pKD46 in NM, but nothing grew, implying that the problem is with the NM medium.
- There were small colonies on the MAGE round 2 amp plates, so we chose 12 from each plate and grew them in 100µL of SOC medium.
- PCR of the colonies for sequencing:
- KAPA mastermix and protocols, with His3_F and His3_internalR primers, 2µL culture as template, 65˚C annealing, 15 sec elongation, 25 cycles
- ran on gel: band around 500-600, but very faint (probably because we did not let the culture grow up enough)
- sent to Genewiz to be sequenced with His3_F primer
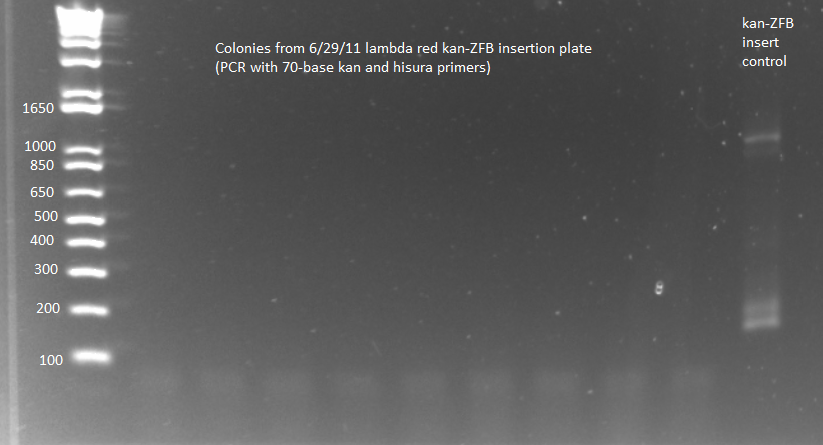
Lambda Red recombination results:
- The plates from 6/30 had an almost crystalline formation on them, which may or may not be bacteria. The 6/29 plates, which we had left at 30˚C, did have colonies but they may be contaminants. We grew up 12 colonies from each 6/29 plate and 6 "colonies" from the 2mL 6/30 plate in SOC medium with kanamycin, and next week we will PCR the 1529620 locus to check.
pZE21G spec backbone:
- PCR of backbone was successful (see E gel below). Gel extracted with Qiagen kit with the following modifications to the kit instructions:
- 2 gel volumes of buffer QG, heated 10-15 min
- after heating added 10µL 3M NaOAc
- eluted in 30µL ddH2O
- 157.4ng/µL 260/280=1.9; 129.2ng/µL 260/280=1.88
Selection system plates:
- Made 3-AT and 5-FOA plates following 2006 Meng Nature Protocol (see protocols for details). 5-FOA plates have a blue stripe, 3-AT plates (0.5 mL 3-AT) have a green stripe.
July 4th
FIREWORKSJuly 5th
Team ZF Assembly
- Assemble and confirm zif268 plasmid
- Lac-ω-zif268 with spec → Lac-zif-ω with cpic
- ZF: 2-3 ultramers
- PCR out ω subunit
Team Wolfe Selection
Goals for the week:
- MAGE out HisB
- What we've been trying/why it's not working — keep trying!
- PCR out Kan Cassette (colony PCR)- analysis/check that it worked
- We did the insertion and have things growing in Kan that look like our cells — we probably have it!
- As long as it's the right size and in the right place, it probably worked, meaning we have 2 HIS genes → MAGE is to remove the endogenous genome copy in vivo
- Construct (PSR01): ~ - KAN - ZFbs - weak promoter - URA3 - HIS3- ~
- Fix/Test NM media
- Made last week, nothing worked in it — try more glucose
- Wolfe strain seems to grow really slowly (~36h); try glycerol stock of PKD46, grows faster
- Make other 3-AT plates (1.5)
- 3-AT is a competitive inhibitor of His; the stronger His is expressed, the more 3-AT the cell can tolerate and still grow
- Test 3-AT plates
Today's results:
- PCR to check whether kan-ZFB-wp-hisura was successfully inserted into the genome
- Used KAPA master mix and protocols, same procedure as 6/29/11
- Because of KAPA shortage, only ran samples 6-8 from 100µL 6/29 plate and 6-7 from 2mL 6/29 plate plus (ΔHisB)ΔpyrFΔrpoZ+pKD46 from 6/29 culture as a control (should produce a band around 300bp, while the insert would make the band 2-3kb)
- Ran on E-Gel with team TolC's samples, but there was an issue loading the gel, so also ran separately on another gel. Unfortunately most of the PCR reactions strangely did not work, but two of the samples appeared not to have the insert (see below).
- Redo PCR:
- out of KAPA so had to use Invitrogen Platinum Supermix
- each reaction had 22.5µL supermix, 0.5 µL each 10µM primer, 1µL template, 0.5µL H2O
- 20 reactions, including one from the same control used above.
- followed Invitrogen instructions, with 3 min elongation, 56 C annealing, 30 cycles
- Assuming the PCR is unsuccessful, we will grow up (ΔHisB)ΔpyrFΔrpoZ+pKD46 (from 6/28 plate) overnight in LB+amp
- NM medium:
- To test Sarah's NM (made 6/30) we will grow up pKD46 strain in 3mL of it overnight at 30 C. If no bacteria grew, we will try remaking it ourselves.
Team TolC
- PCR out and insert Kan construct in front of TolC (similar to Wolfe selection)
- Construct 1: ~ - KAN - ZFbs - weak promoter - ~ (done, PCR, gel extraction)
- Used primers ZFB-wp-tolC-r and tolC-kan-f
- Construct 2: ~ TolC ~ (do not have primers yet)
- Note that ~ indicates a homology region
- Construct 1: ~ - KAN - ZFbs - weak promoter - ~ (done, PCR, gel extraction)
- Need the EcNR2 strain to come in before we can work with it (came in Thursday 7/7/2011, the one from Wednesday 7/6/2011 was not growing well.)
- Inactivate rpoZ (similar to inactivating His3, but should be easier to use MAGE with- EcNR2 is a strain designed for MAGE)
- MAGE oligo rpoZ deletion will arrive on Thursday 7/7/2011
- Make SDS (how we test TolC +vely)(done, stored at room temperature)
- Only cells with active TolC can survive SDS treatment
Web Design
To Do
- Safety Description
- Email Alain/Harvard biosafety group ✔ (Meeting pending)
- Needed information:
- Strain names
- Antibiotic Resistance
- DNA inserts/removals
- Draft Project Description ✔
- Finalize
- Update the Facebook page/create a new 2011 page
- Get the Public Wiki up and running
- Figure out templates
- Update Twitter
Notes to All Teams
- Teams: Make sure everything is organized in your dropbox!
- *Announcing a New (and Final) Plasmid Name* PSR01: formerly known as Wolfe Plasmid/Vatsan's Plasmid/Selection
- Contains ~ - KAN - ZFbs - weak promoter - URA3 - HIS3- ~
July 6th
Team ZF Assembly
Because our primers still haven't arrived, the members of Team ZF Assembly are working with the Web Design Team.
Team Wolfe Selection
- Redo PCR Results:
- Samples 1,3,4,5,6,7,8,9,11 from the 100µL 6/29 plate all showed unsuccessful introduction of kan-ZFB-wp-hisura
- Samples 2,3,4,6,7,8,9,10,11,12 from the 2mL 6/29 plate all showed unsuccessful introduction of kan-ZFB-wp-hisura
- (ΔHisB)ΔpyrFΔrpoZ+pKD46 from 6/29 culture used as a control
- NM media has 1% of the glucose concentration written in the protocol, it is 0.4mg/ml instead of 40mg/ml. Nevertheless, our overnight culture of pKD46 grew successfully so we will continue to use the media unless other problems arise.
- One possible explanation for our failure to PCR out a kan-ZFB insert from the recombined colonies is that the insert was integrated into the genome somewhere else besides the 1529620 locus. This might be why the PCR shows no insert while the colony still grows in kanamycin. We will try to PCR the insert using primers that anneal to the kan and his-ura sections (since these primers are about 70 bases long, we will do a 2-step cycle)
- Invitrogen Platinum Supermix reagents and protocols, with 3:30 at 72˚C instead of separate annealing and elongation, 25 cycles
- used same cultures as for PCR listed above
- kan-ZFB-wp-hisura insert as control
- results from 2 E Gels: PCR not very successful. Colonies showed no bands while the control showed only a side product too small to be the full insert.
- Grew up 24 more kan-ZFB lambda red recombined colonies (12 from 100µL 6/29 plate, 12 from 2mL 6/29 plate) in LB+kan in 96 well plate for PCR tomorrow
- Diluted 24 colonies (1 ss+pKD46 control, 11 from MAGE round 2 1µL plate, 12 from MAGE round 2 10µL plate) in 30µL ddH2O and split between NM,NN+Histidine, and LB+amp to check his phenotype
- NM+his media: 49mL NM, 1 mL 5% histidine solution
- Made 1.5mL 3-AT plates
Team TolC
- Gel extraction of KAN-ZFBs-wp as there is a side product.
- To increase yield, spin down the contents of both eppendorf tubes into a single column, have a 2:1 volume ratio of QG Buffer to gel volume, e.g. 1ml QG buffer to 0.5ml gel in step 2, and elute in 30µl water in step 13)
- checked the ECNR2 strains, both appear to have not grown
- note from 7/7/2011, new ECNR2
- running another PCR since there was errors in gel extraction, and the yield was less than 20ng/µl, and purity was very bad.
TOLCINV PCR-Machine 7
- 94°C for 2 min
- 94°C for 30 s
- 53°C for 30 s
- 72°C for 1.5 min
- Previous three steps repeat 30 times
- 72°C for 5 min
- 4°C forever
Web Design
- Project description
- Web design brainstorming;exploring past designs and templates
 "
"