Team:Paris Bettencourt/Modeling/Assisted diffusion/From membrane tension to liquid flux
From 2011.igem.org

From membrane tension to liquid flux
To deduce the force exercising on the tube from membranian pressure, please refer to an explanation figure below :
 |
 |
| Fig1: Tension-force_explanation / The force applied to the nanotube as a function of the surface tension force. | Fig2: Geometry / The system geometry. state during the steady state phase. |
Here T is a surface tension, N is a resulting force of the area of the tube attachment to the bacterium, Δα or α is the half of the angle with a vertex in the center of the bacterium and between the tube extremities. Only this zone is giving a non-compensated contribution to the resulting force.
So the force exercising on the tube is given by the formula :








Getting good parameters
We have done several simulations where the system consisted of two spherical bacteria of radius R1 and R2, the nanotube of the length L and the radius r between them, and the number of phospholipids N1 and N2 on them. The nanotube dimentions we have taken from the article published by Dubey and Ben-Yehuda [1] (r = 100 nm, L = 1 µm). The ideas about what values to take for R1, R2 and N1, N2 we got from the bionumber's site <a href="https://2011.igem.org/Team:Paris_Bettencourt/Modeling/Assisted_diffusion#references"> [2]</a>. We have checked several sets of parameters :
- N1 = 2*106, N2 = 1*106, R1 = R2 = 0.5 µm;
- N1 = 1*107, N2 = 5*106, R1 = 2 µm, R2 = 1 µm;
The value of dynamic viscosity of phospholipid bilayer taken is 10 Pa.s, a bit more than what can be found for castor oil.
Analysing the results and conclusion
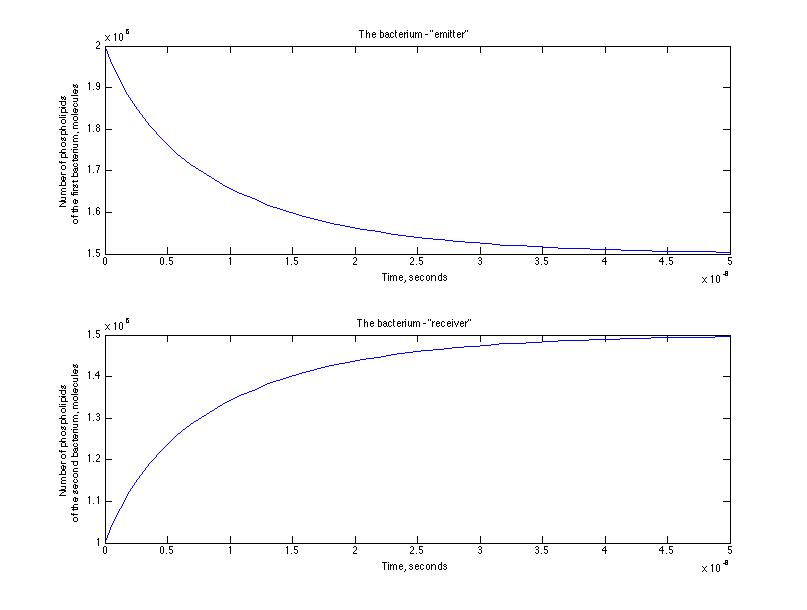
Here is the representation of the cinetic between the emitter and receiver - the number of phospholipids passed from the emitter to the receiver through the nanotube.
After a set of simulations we have estimated the characteristic time of the process and it was of the order of 10-8 seconds for the first simulation and 10 -6 seconds for the second one. That means that we were right at the beginning that an assisted diffusion process is much faster that a passive diffusion.
The masses transported are of the order of 10 -18 kg, which is an interesting result. In terms of the volume exchange : we can observe that about 10 -21 m 3 of liquid is transfert from the emitter to the receiver. 0,1 % of the emitter volume is transported due to an assisted diffusion. This doesn't seem much and it certainly doesn't entirely explain the original gfp diffusion experiment. It is nevertheless interesting to see such orders of magnitude.
Anyway this process can assist the passive diffusion in a way that if we have a lot of molecules in one bacterium that are localized near the membrane and nanotubes, it may accelerate the diffusion.
We still have to work on this model in order to tinker with some of the problem parameters. This might permit us to see a faster and more significant molecule transport through the nanotubes.
References
- Intercellular Nanotubes Mediate Bacterial Communication, Dubey and Ben-Yehuda, Cell, 2011, available <a href="http://bms.ucsf.edu/sites/ucsf-bms.ixm.ca/files/marjordan_06022011.pdf">here</a>
- BioNumbers <a href="http://bionumbers.hms.harvard.edu/">here</a>
</html>
 "
"