Cyrille
Going on with the QCM experiments
The transformation have worked pretty well. The negative control before digestion has some clone, and the negative control after digestion has none. On all the plate, we have a important number of colony, more important than in any negatives control. This indicate that the PCR may have worked.
On the selected clones, the last two (11, and 12) comes from very small colonies on the plate. The hypothesis is that they might be parasite, or clones grown with a non metilated plasmid. Anyway, the yield obtained after the miniprep is poor, and they may come from parasite.
The miniprep was done over 12 selected clones. The culture was grown overday, started at 9:30 and centrifugated at 15:00. The yields are around 500ng/µL depending on the the tubes. The concentration was measured with the Tecan machine.
1µg of each of the 12 clones (except for the last two, from which we took the maximum quantity that is to say 8 µL) was digested using EcoRI-Fast digest in green digestion buffer, for 15 min at 37°C. The result was loaded on the gel, and let run for 20 min at 50V and then at 100V for the rest. Here is the result of yhe gel:

Ladder - 1 - 2 - ... - 12 - ladder
The result is again negative. There are two bands at 3kb indicating the plasmid still have two restriction sites.
We will start again tomorow with Ariel menages digestion protocol...
Kevin
Culture of double transformed cells (suite)
Diluted at 1/100 => expected to be between OD 300 and 600
- 3 Tubes pSG20 at 37°C
- 3 Tubes pFX234 at 37°C
- 3 Tubes pSG20 only at 30°C
- 3 Tubes FX234 only at 30°C
- 3 Tubes pSG20/pDAG464 at 30°C
- 3 Tubes pFX234/pDAG479 at 30°C
At OD 300-600, induction of fusion protein with arabinose 0%, 0,1 % and 0,2% for 1h30.
Microscopy (suite)
37°C
LacI:GFP 37°C
LacI:GFP protein fusion : 37°C
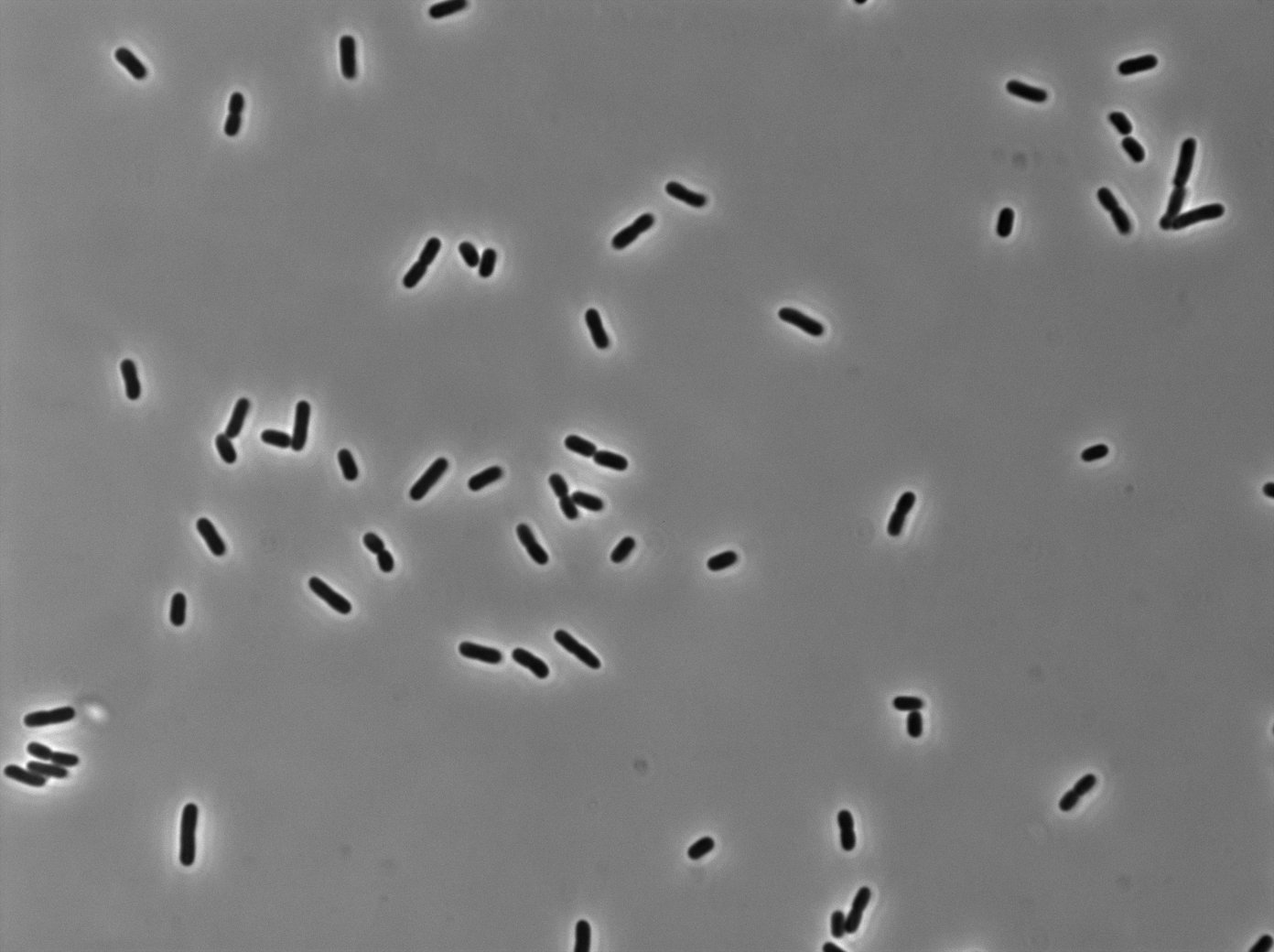 LacI:GFP protein fusion induced with no arabinose on E. Coli from Dave Lane plasmids. |  LacI:GFP protein fusion induced with no arabinose on E. Coli from Dave Lane plasmids. |
 LacI:GFP protein fusion induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |  LacI:GFP protein fusion induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |
 LacI:GFP protein fusion induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. | 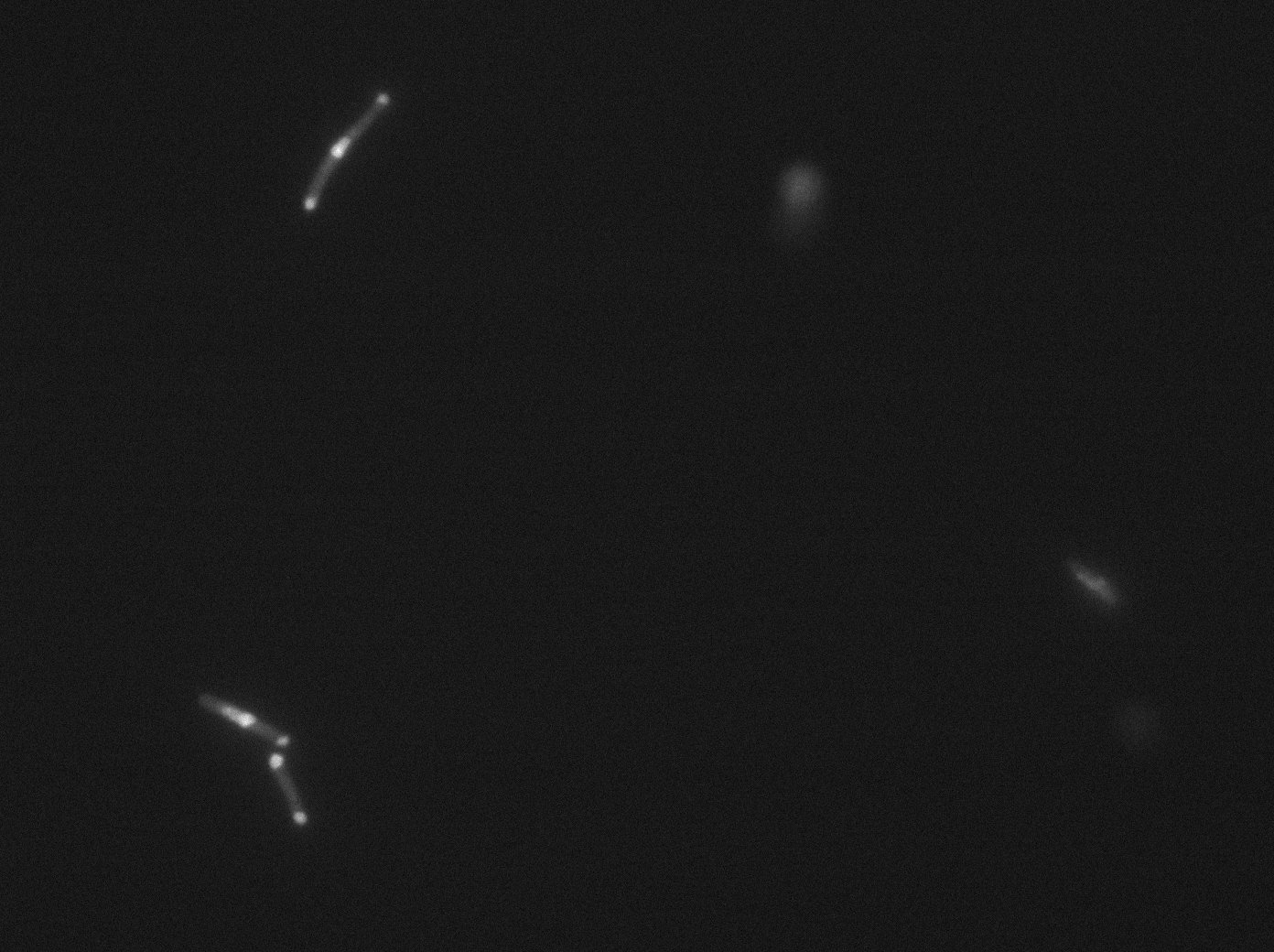 LacI:GFP protein fusion induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. |
LacI:GFP / Lac O array 37°C
LacI:GFP /LacO array : 37°C
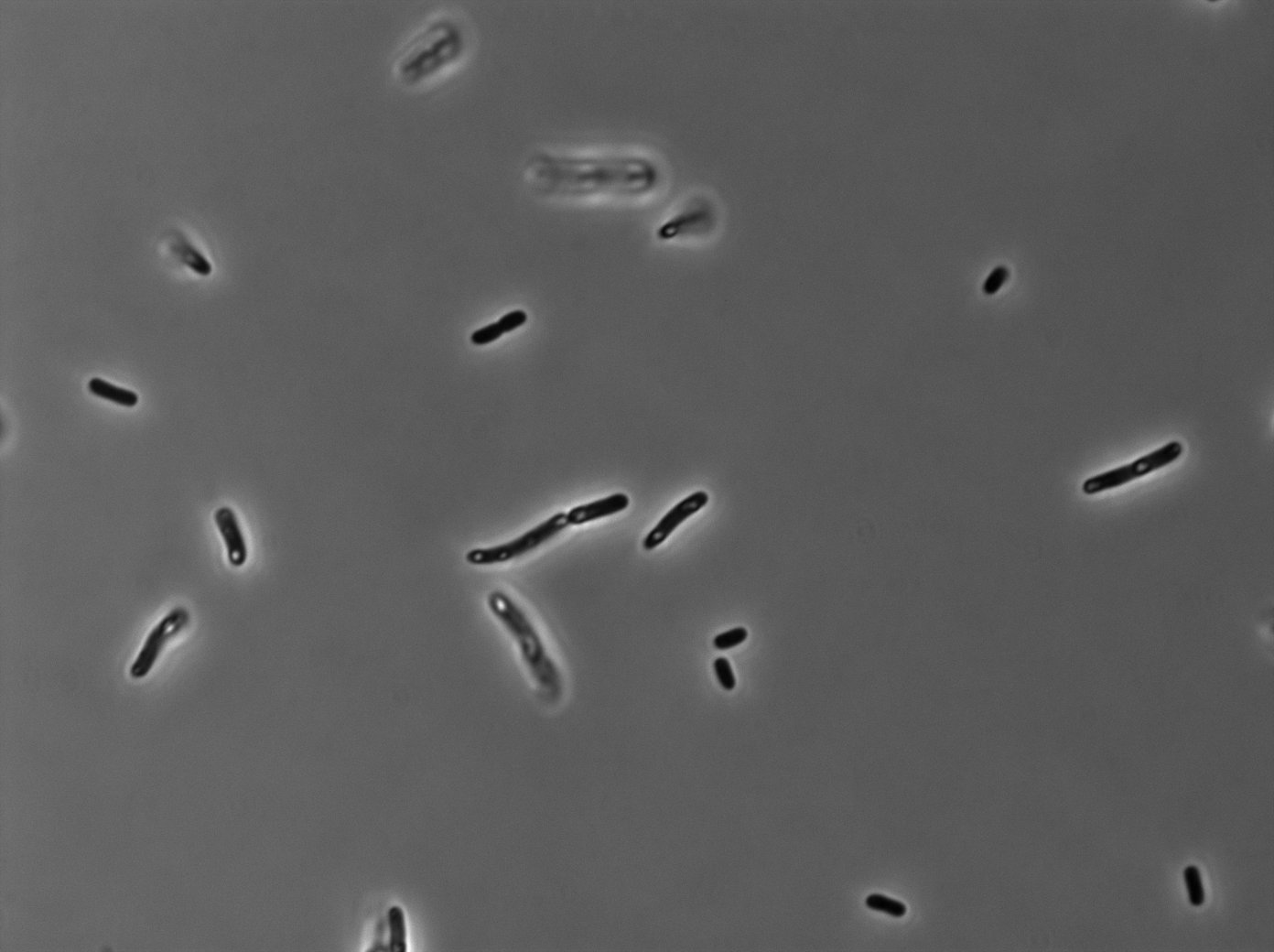
 GFP:LacI / LacO array induced with no arabinose on E. Coli from Dave Lane plasmids. |  GFP:LacI / LacO array induced with no arabinose on E. Coli from Dave Lane plasmids. |
 GFP:LacI / LacO array induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. | 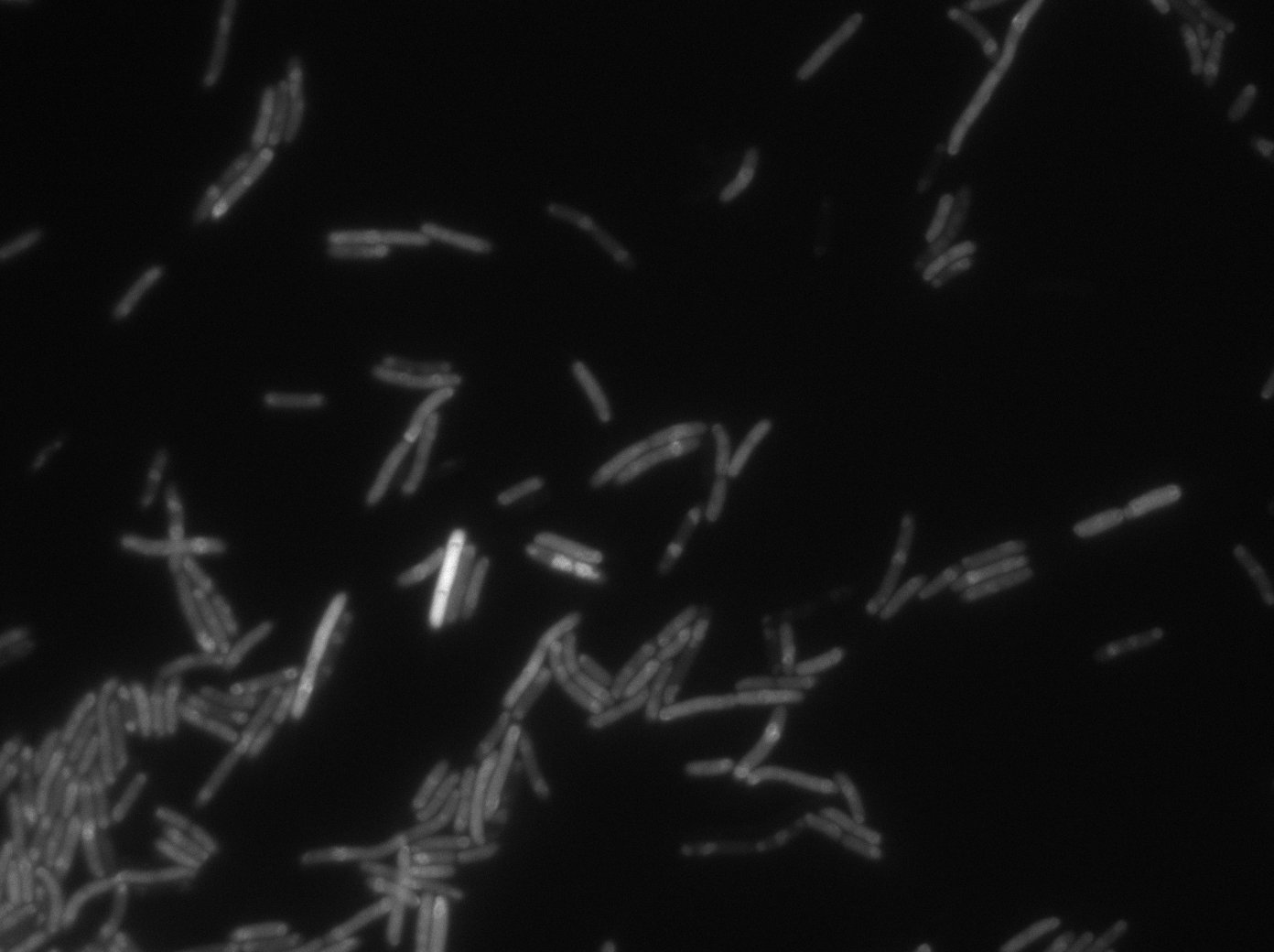 GFP:LacI / LacO array induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |
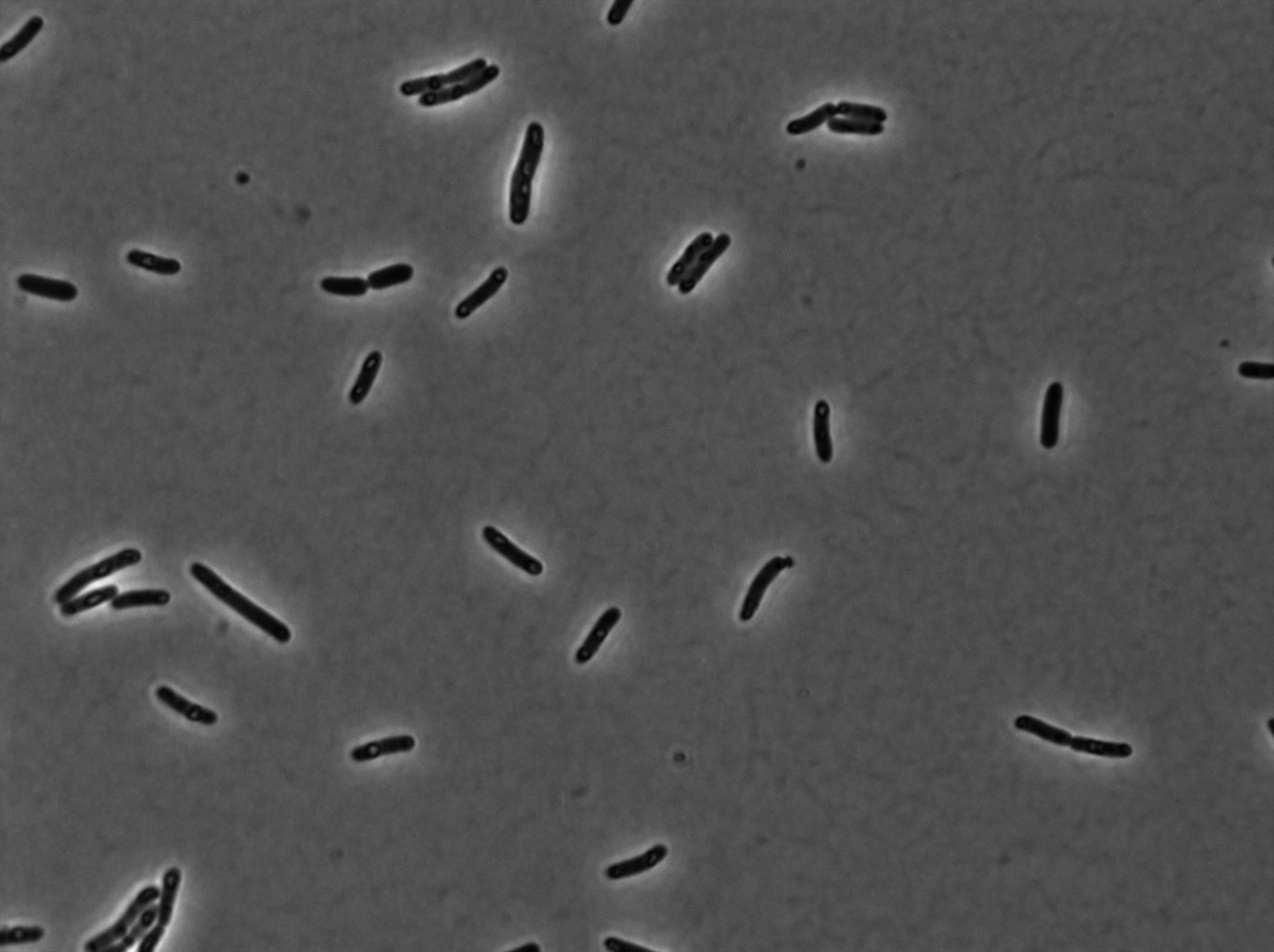 GFP:LacI / LacO array induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. | 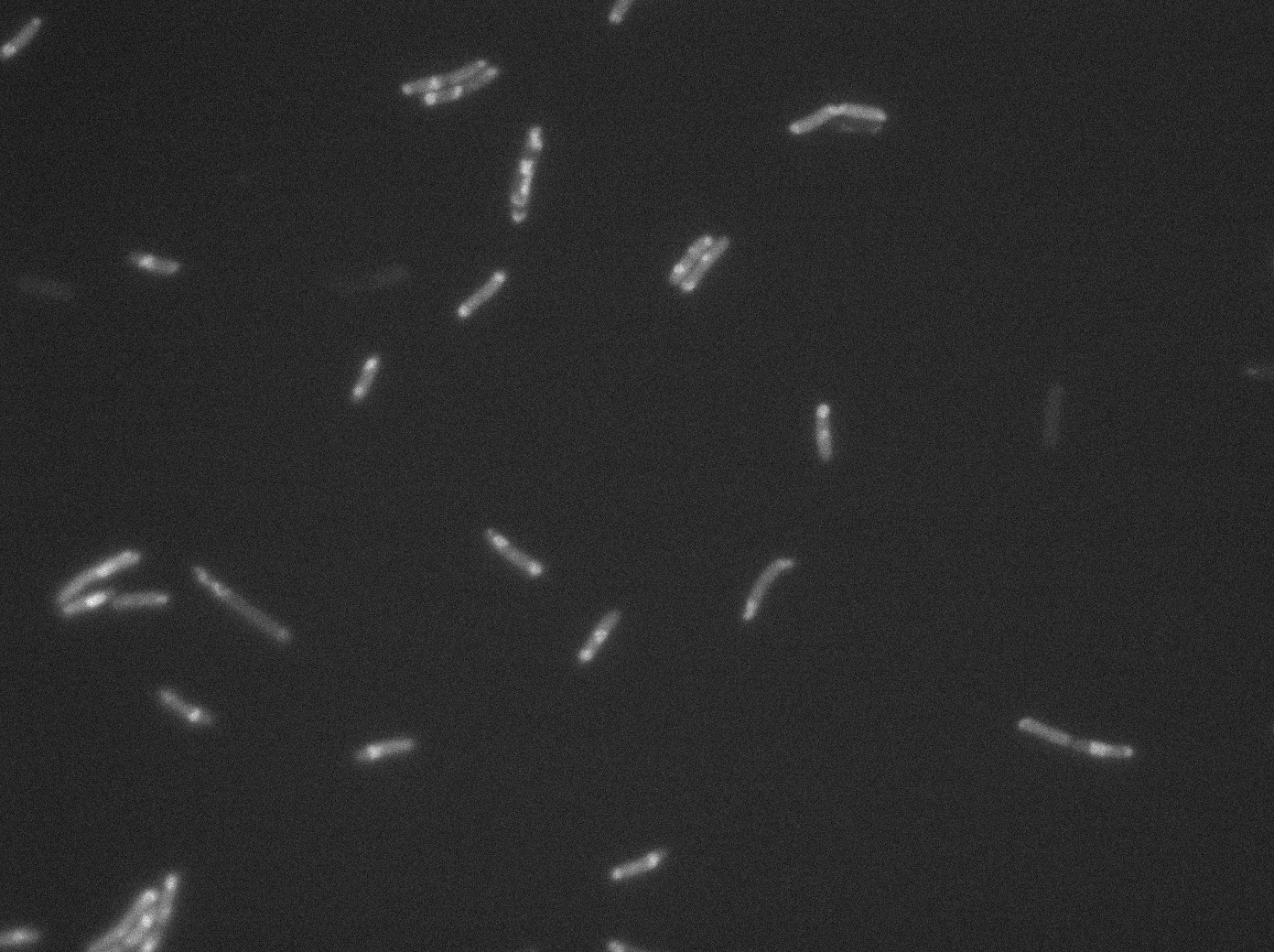 GFP:LacI / LacO array induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. |
tetR:YFP 37°C
tetR:YFP protein fusion : 37°C
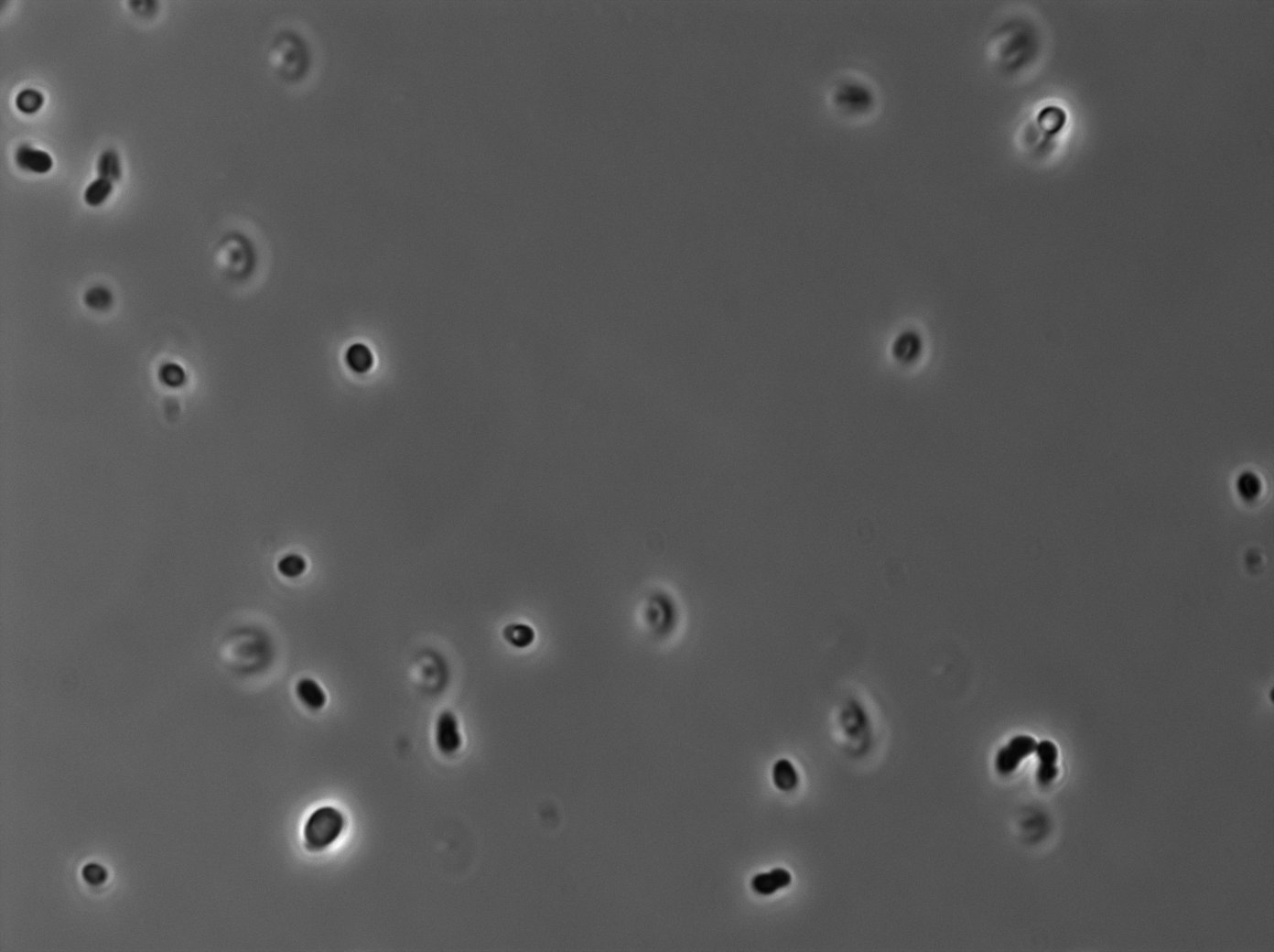 tetR:YFP protein fusion induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |  tetR:YFP protein fusion induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |
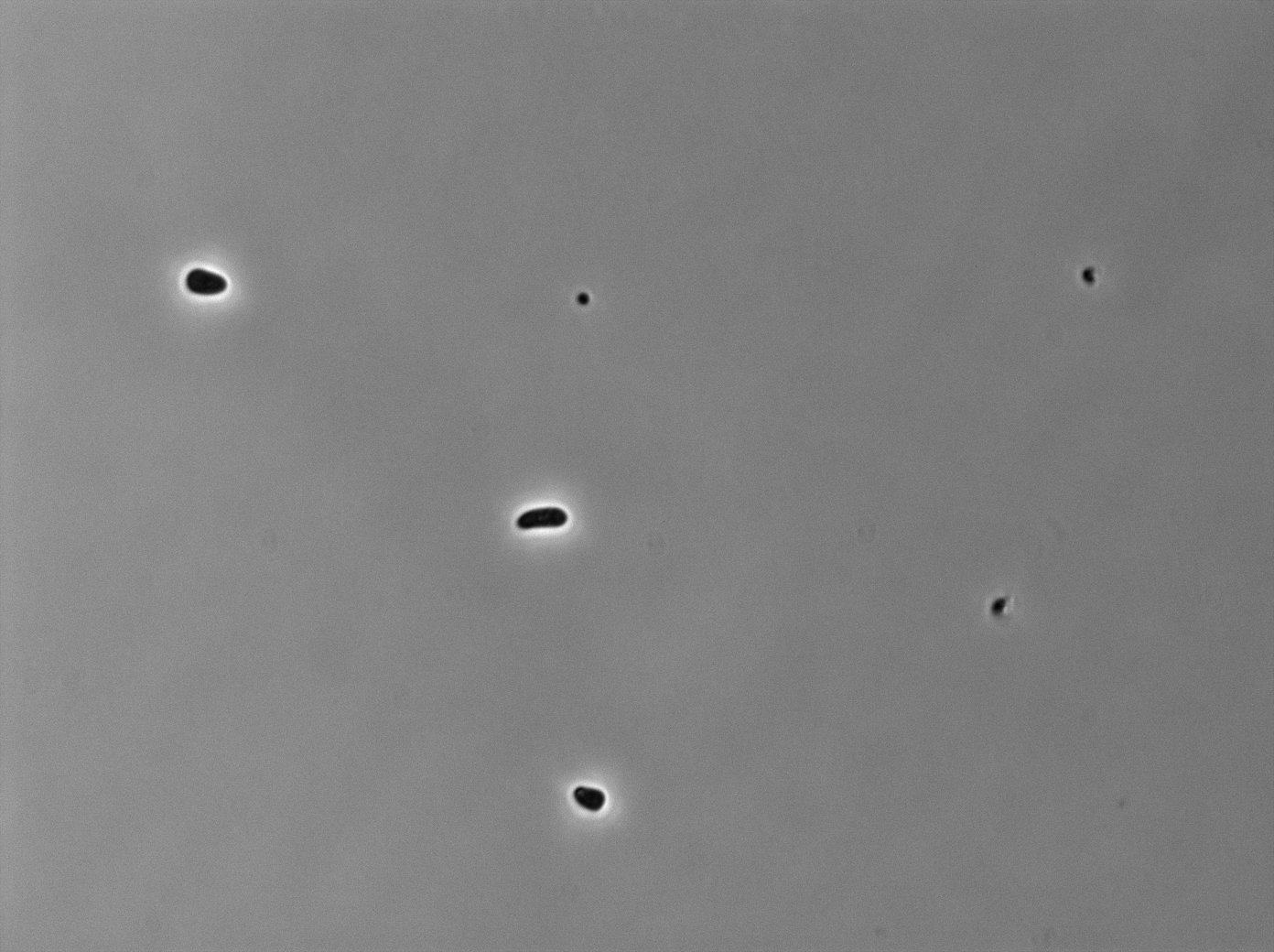 tetR:YFP protein fusion induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. |  tetR:YFP protein fusion induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. |
tetR:YFP / tetO array 37°C
tetR:YFP / tetO array : 37°C
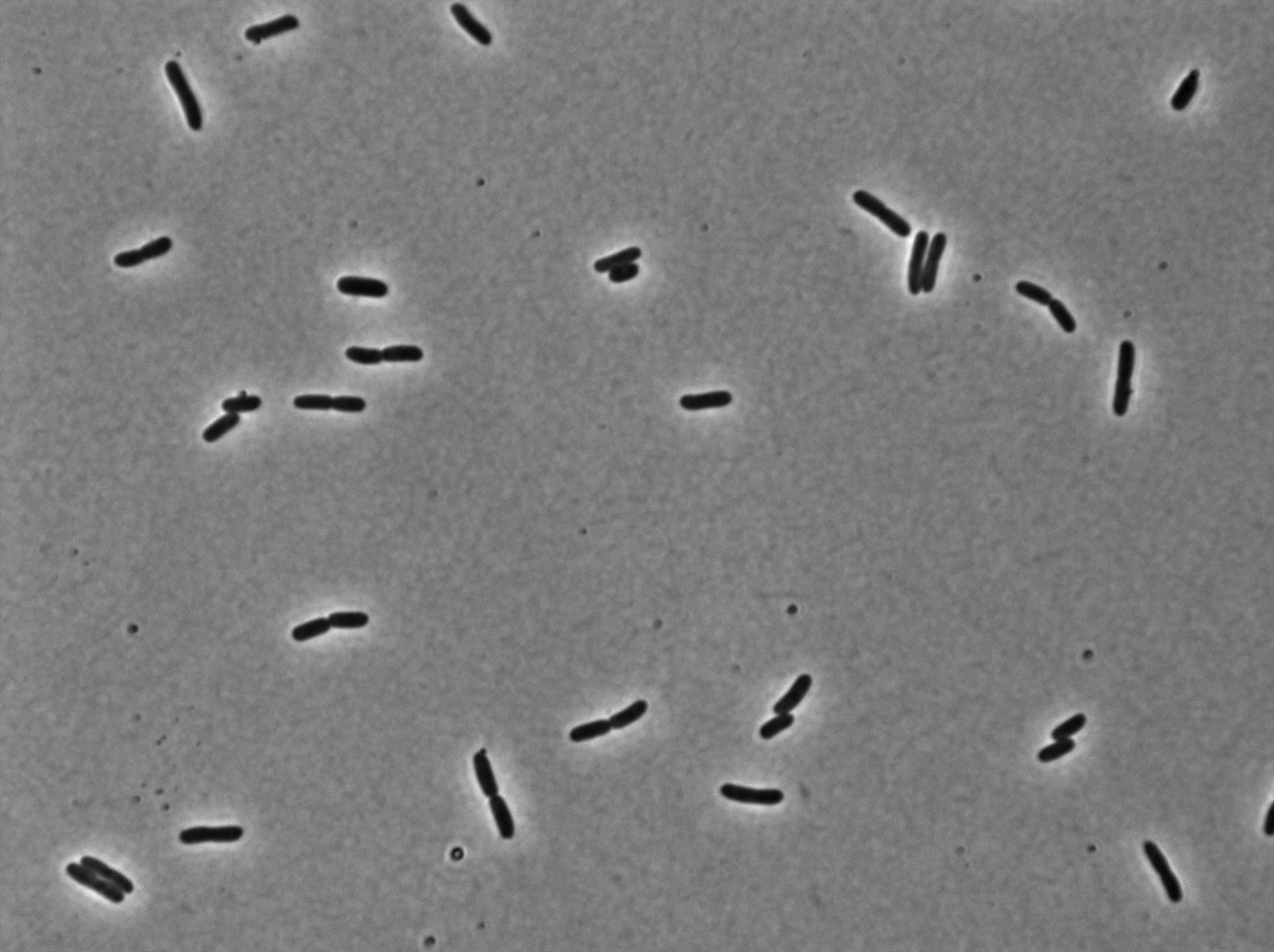 tetR:YFP / tetO array induced with no arabinose on E. Coli from Dave Lane plasmids. |  tetR:YFP / tetO array induced with no arabinose on E. Coli from Dave Lane plasmids. |
 tetR:YFP / tetO array induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |  tetR:YFP / tetO array induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |
 tetR:YFP / tetO array induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. |  tetR:YFP / tetO array induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. |
We can see lots of protein fusion agregated at 37°C unfortunately. More in Laci than tetR.
30°C
LacI:GFP 30°C
LacI:GFP protein fusion : 30°C
 LacI:GFP protein fusion induced with no arabinose on E. Coli from Dave Lane plasmids. |  LacI:GFP protein fusion induced with no arabinose on E. Coli from Dave Lane plasmids. |
 LacI:GFP protein fusion induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |  LacI:GFP protein fusion induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |
 LacI:GFP protein fusion induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. |  LacI:GFP protein fusion induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. |
LacI:GFP / Lac O array 30°C
LacI:GFP /LacO array : 30°C
 GFP:LacI / LacO array induced with no arabinose on E. Coli from Dave Lane plasmids. |  GFP:LacI / LacO array induced with no arabinose on E. Coli from Dave Lane plasmids. |
 GFP:LacI / LacO array induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |  GFP:LacI / LacO array induced with 0,1% arabinose on E. Coli from Dave Lane plasmids. |
 GFP:LacI / LacO array induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. |  GFP:LacI / LacO array induced with 0,2% arabinose on E. Coli from Dave Lane plasmids. |
tetR:YFP 30°C
tetR:YFP protein fusion : 30°C
 tetR:YFP protein fusion inducted with no arabinose on E. Coli from Dave Lane plasmids. |  tetR:YFP protein fusion inducted with no arabinose on E. Coli from Dave Lane plasmids. |
 tetR:YFP protein fusion inducted with 0,1% arabinose on E. Coli from Dave Lane plasmids. |  tetR:YFP protein fusion inducted with 0,1% arabinose on E. Coli from Dave Lane plasmids. |
 tetR:YFP protein fusion inducted with 0,2% arabinose on E. Coli from Dave Lane plasmids. |  tetR:YFP protein fusion inducted with 0,2% arabinose on E. Coli from Dave Lane plasmids. |
tetR:YFP / tetO array 30°C
tetR:YFP / tetO array : 30°C
 tetR:YFP / tetO array inducted with no arabinose on E. Coli from Dave Lane plasmids. | 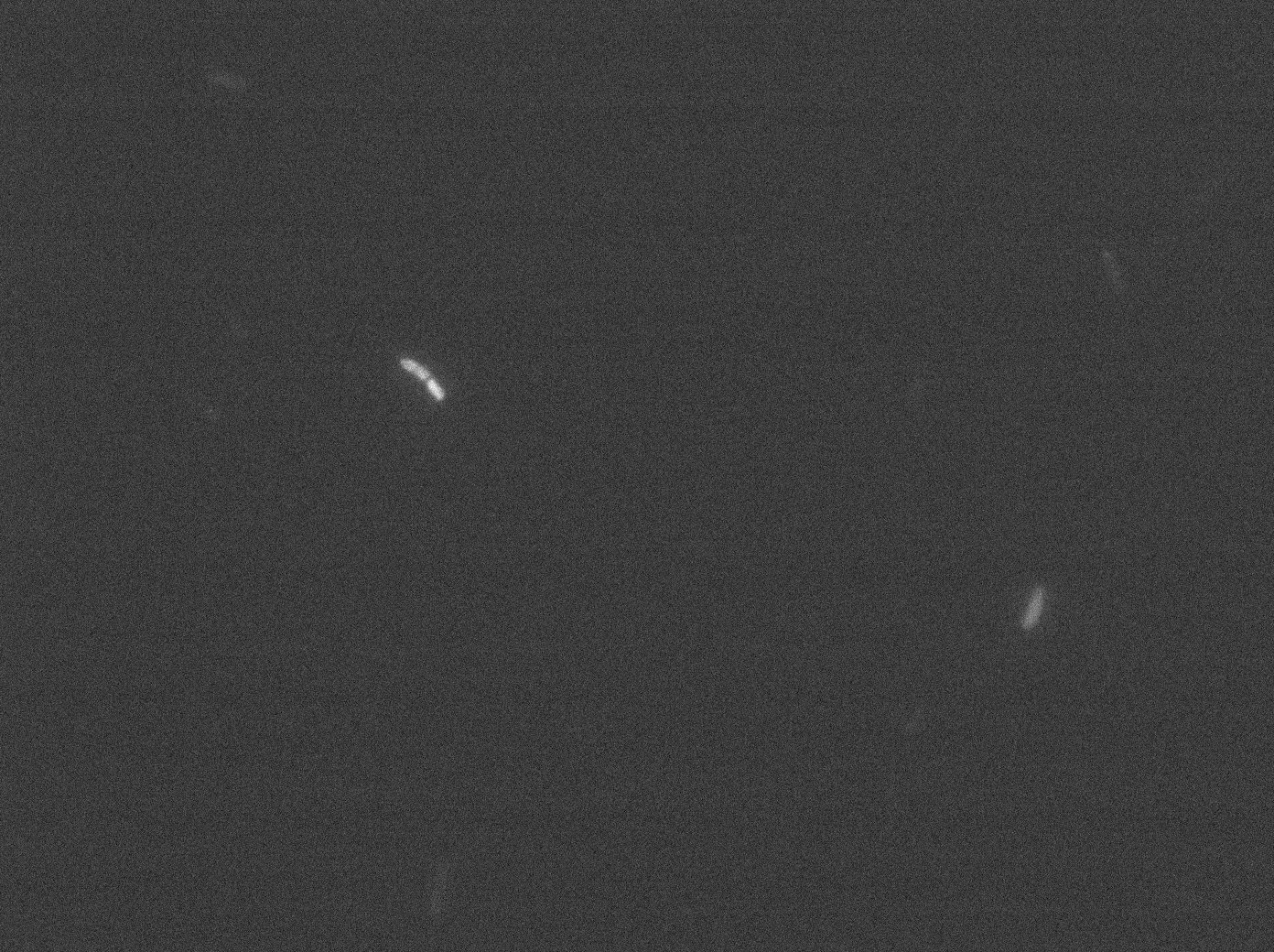 tetR:YFP / tetO array inducted with no arabinose on E. Coli from Dave Lane plasmids. |
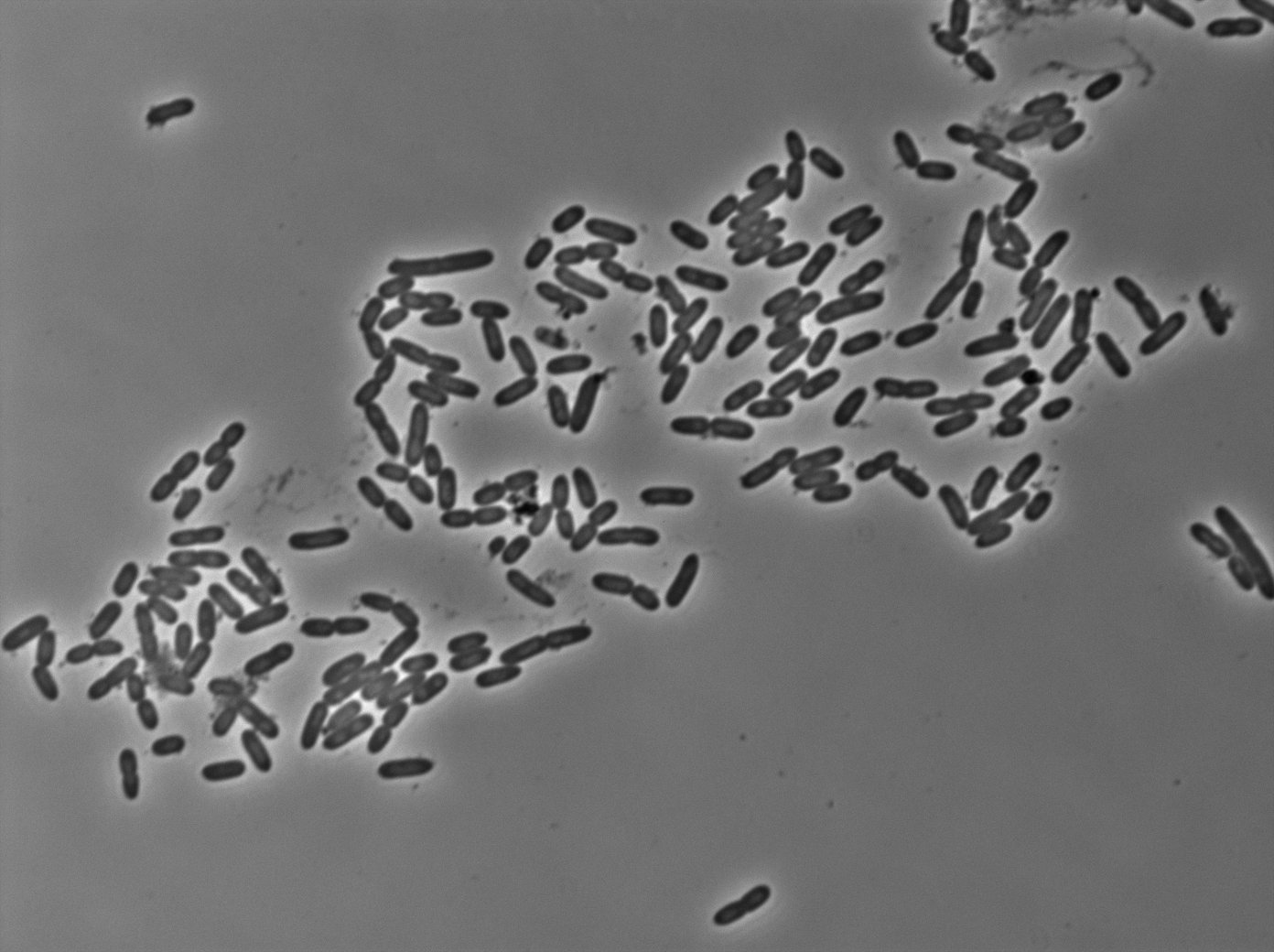 tetR:YFP / tetO array inducted with 0,2% arabinose on E. Coli from Dave Lane plasmids. |  tetR:YFP / tetO array inducted with 0,2% arabinose on E. Coli from Dave Lane plasmids. |
Same observation but less expression in 30°C.
I will do a ladder of induction with arabinose between 0% and 0,1% to see if we can manage to not have agregation.
Gel purification of PCR Phusion
Gel with TBE + 1% Agar.
Digestion X-S:
- 2uL 10X Fast Digest Green Buffer
- 1uL XbaI Enzyme
- 1uL SpeI Enzyme
- 1ug for each plasmid (pSG20 - pFX234 - pDAG464 - pDAG479 - S27)
- qsp 20uL
15min in 37°C
Loading on gel with 3uL DNA Ladder 1kb
Electrophoresis 50V for 30min

Gel of Dave Lane plasmids digestion
Digestion for S27 works. Didn't for Dave Lane plasmids.
Protocol of qiaQuick gel extraction for S27 plasmid.
Danyel & Camille
tRNA
The 5th colony of the tRNA transformation was named strain S38.
A glycerol of this strain was prepared and stored. (labelled S38)
The miniprep of S38 and S21 were prepared. They were then digested on site S & P and X & P respectively.
The digestion product of S21 was gel extracted whereas the S38 digestion product was treated by PCR purification. (8.6 ng/µL and 12.8 ng/µL)
The ligation mix held a insert/vector ratio of 10:1. The total volume was 10mL.
2 mixes were prepared: one for the ligation and a negative control.
The mix was incubated at 22ºC overnight to be transformed early tomorrow morning.
Edward
Overnight cultures (LB)

 "
"















































