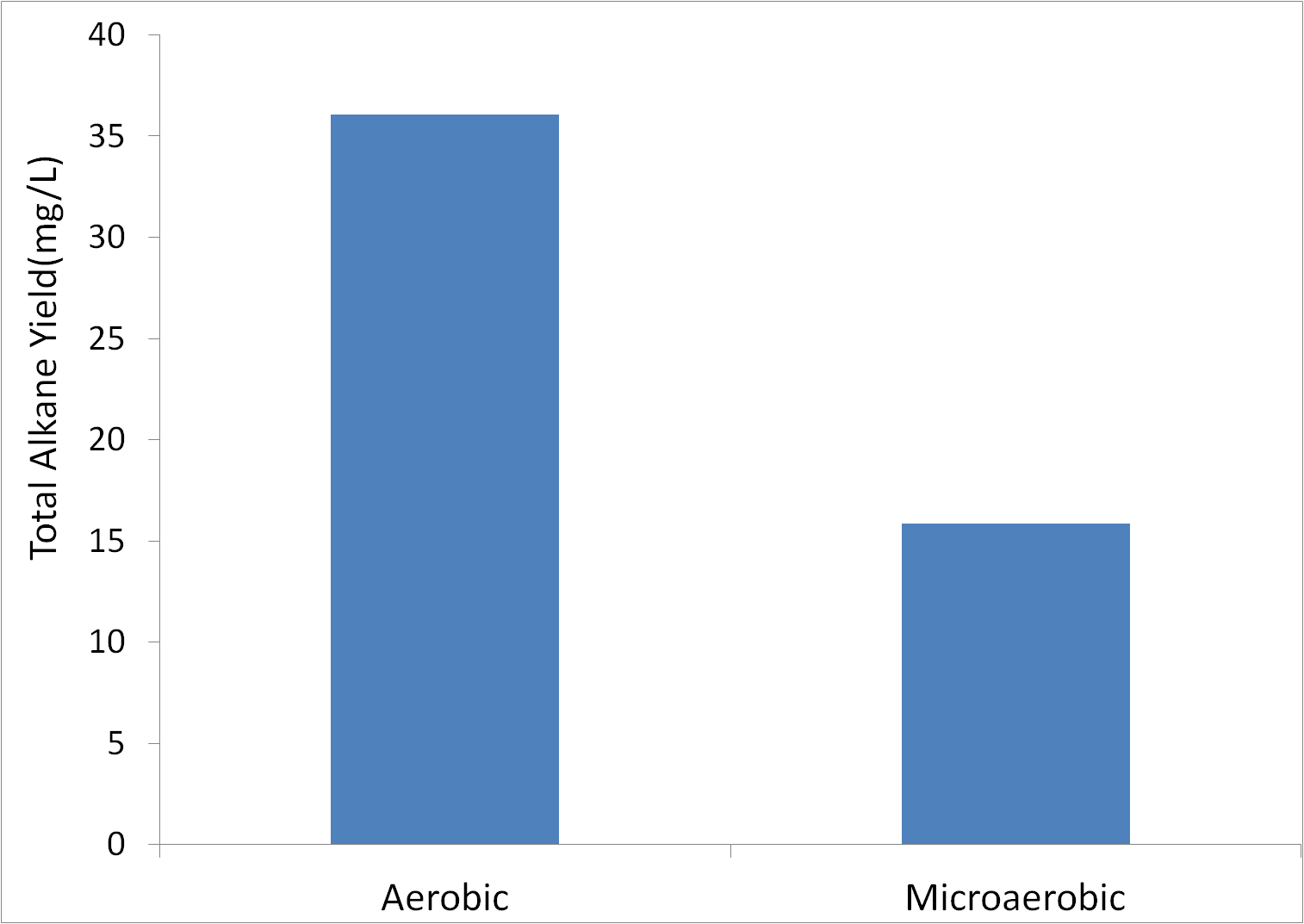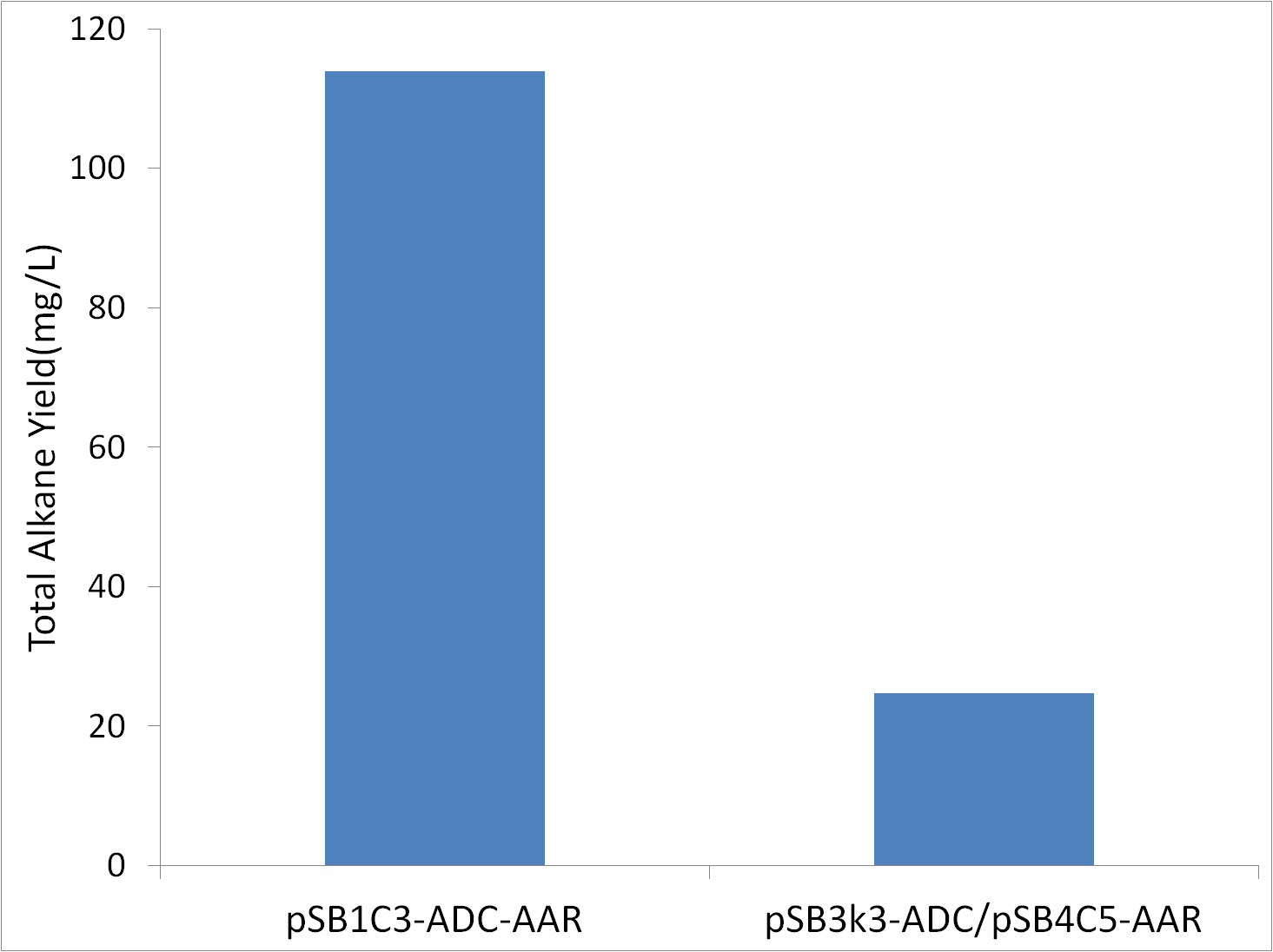Team:Washington/Alkanes/Future/Vector
From 2011.igem.org
System Optimization
Using our initial, non-optimized growth conditions, we were able to obtain alkane/ene yields of approximately 2 mg/L. In order to make analysis easier, and to make it easier to determine the effects of the addition of additional modules, we wanted to increase yield. Our efforts focused on how by varying system conditions, we could increase yield.
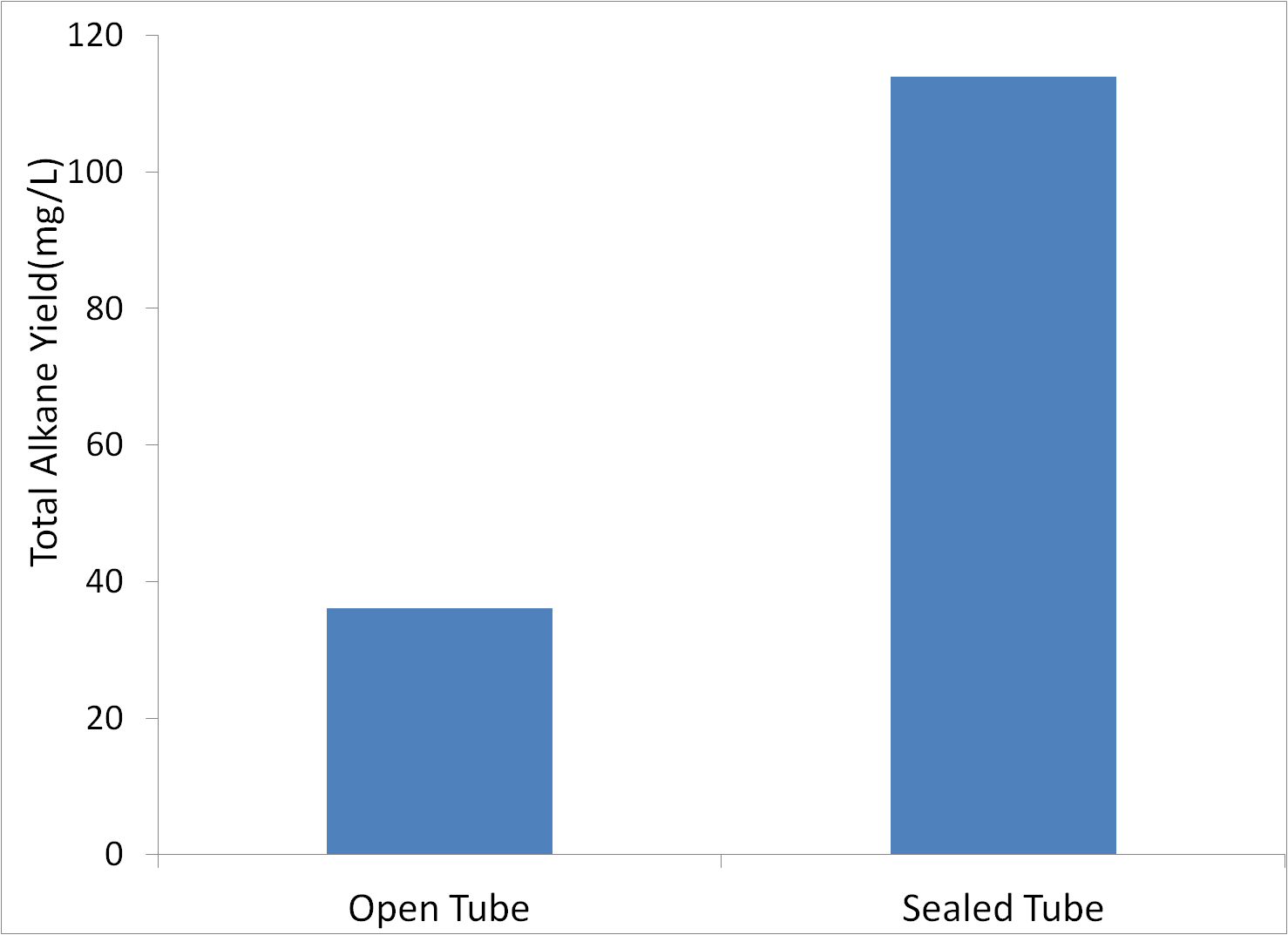
Sealed vs. Open Tubes
We were concerned that produced alkanes may have been evaporating, reducing apparent yield. Therefore, we performed tests where the tubes were either capped the standard way, or capped with foil coverting the opening, thereby reducing evaporation. Tests were conducted in glass tubes with M9 production media, with MG1655 innoculated to an initial OD600 of 1.
Covered tubes showed significantly more alkanes, so all further tests would be conducted in sealed tubes.
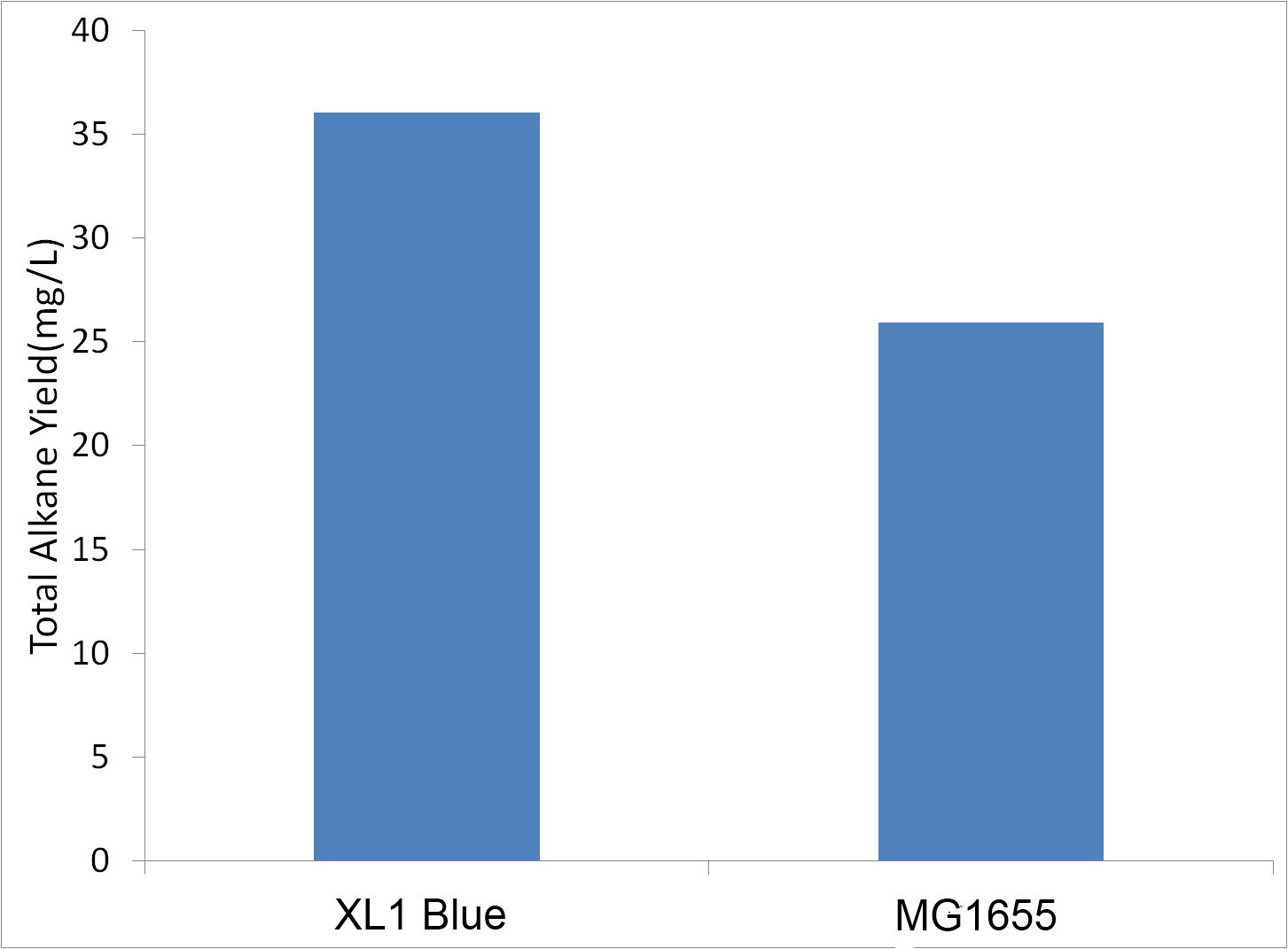
Use of Different Strains
We had suspected that different strains of E. coli would produce varying amounts of alkanes. Initial experiments were done in MG1655, and we decided to test XL-1 blue (a commercial supercopentent variant of DH5a) for the ability to produce alkanes. Tests were conducted in sealed glass culture tubes in M9 production media . Cells were innoculated to an OD600 of 1 when conducting this test.
XL-1 blue was able to produce more alkanes than MG1655. Therefore, future tests were conducted in XL-1 Blue.
Aerobic vs Microaerobic Growth
In many industrial production applications, growth in conditions with little or no oxygen can improve yield. Therefore, we tested alkane yield in cultures grown in airtight vials with only a small amount of air on the top in order to severely limit oxygen availability. Tests were conducted in glass (note that the test was conducted in open culture tubes for aerobic cultures, and sealed glass vials for microaerobic cultures, with M9 production media in XL21-blue cells.)
Based upon this data, aerobic growth appears to be better for alkane yield. Note that this difference is not likely due to differences in growth rate, as both cultures were inoculated to a high OD600 of 1. The fact that this tube was not covered means that the actual alkane yield (before evaporation) was likely higher. Therefore, microaerobic conditions are unlikely to improve yield.
pSB1C3-ADC-AAR vs pSB3k3-ADC/pSB4C5-AAR
The original study expressed ADC and AAR on seperate low copy number vectors. If we could get high alkane yields using low copy number vectors, the lesser protien and vector production would make the use of a lower copy number vector preferable. Tests were conducted in covered glass culture tubes (aerobic conditions) with M9 production media. Cells were XL1-Blue, and were innoculated to an OD600 of 1.
The fact that high copynumber vector use results in an increase in yield means that high expression levels of ADC and/or AAR are required for high alkane yields.
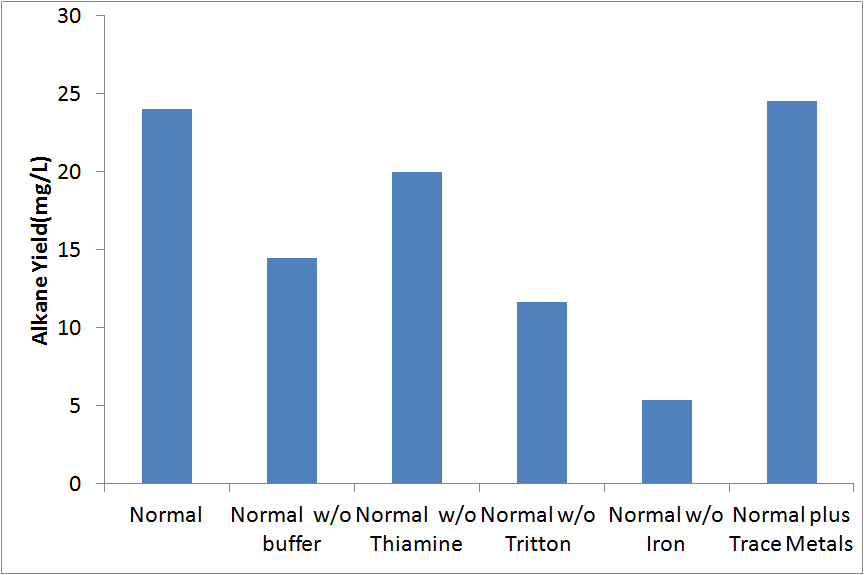
Media composition
Our initial media was based upon the media used in [1]. We did not know if all of the additional components not found in a normal media (buffer, thiamine, Triton, iron) actually improved yield. In order to determine if each of these components imporved yield, we analyzed cultures inoculated into M9 medias without each of these 4 compounds. In addition, we tested media with additional trace metals. These tests were conducted in covered glass culture tubes with XL-1 blue cells.
None of the modifications we made resulted in a significant increase in yield. Therefore, no further modifications to the media used was made.
Effects of Changing Initial Cell Density
Earlier tests were done with an initial OD600 of 1. However, we had never established what the optimal initial OD would be. If significant alkane production occurs during stationary phase, inoculation to a high initial OD may increase alkane production. For this test, we inoculated XL-1 blue (to a final OD600 in a range of 0.01 to 10) in M9 production media (in covered glass culture tubes). We extracted from these cultures after 16 hours of growth.
Higher cell density cultures showed significantly higher alkane yields than lower cell density cultures. The increase of alkane yields at cell densities as high as 10 implies that high amounts of alkane production occurs during stationary phase. Therefore, alkane production scales with cell density. In addition, inducing alkane production during stationary phase decreases the amount of glucose going towards cellular growth instead of alkane production.
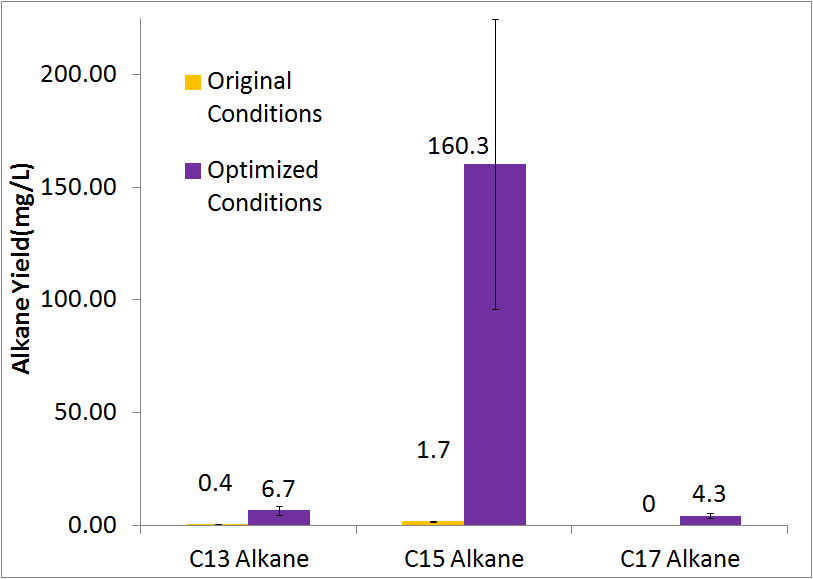
Final conditions
Using our optimized media & growth conditions(XL-1 blue inoculated to an OD600 of 10, in M9 media, in closed glass culture tubes) , we were able to improve total alkane yield 80 fold, from approximenly 2 mg/L (after 48 hrs) to approximently 170 mg/L(after 48 hrs).
Production Curve
Ideally, alkane extraction and analysis would occur after all ( or essentially all) of the glucose in the media was used up. However, we didn't know how long it would take for our cells to use up all of the glucose. In order to determine the best time for extraction, we extracted alkanes from M9 production cultures that had been producing for 6, 24, 48, and 72 hours. Three cultures were used for each timepoint.
The 24 hour timepoint was essentially identical to the 48 and 72 hour timepoints. This implies that our cells are using up 30g/L glucose sometime before 24 hours. Future work will consist of increasing glucose concentration, and increasing inoculated cell density in order to maximize alkane production per culture per day.
References
Microbial Biosynthesis of Alkanes Andreas Schirmer, Mathew A. Rude, Xuezhi Li, Emanuela Popova and Stephen B. del Cardayre Science 30 July 2010: Vol. 329 no. 5991 pp. 559-562 http://www.sciencemag.org/content/329/5991/559
 "
"