Team:EPF-Lausanne/Our Project/Reporter Systems/lacI
From 2011.igem.org
In Vivo Characterization
In vivo Main | TetR-RFP System | TetR-LacI-RFP System | Ptet Characterization | Plac Characterization | Plasmid DetailsTetR - LacI - RFP System
Description
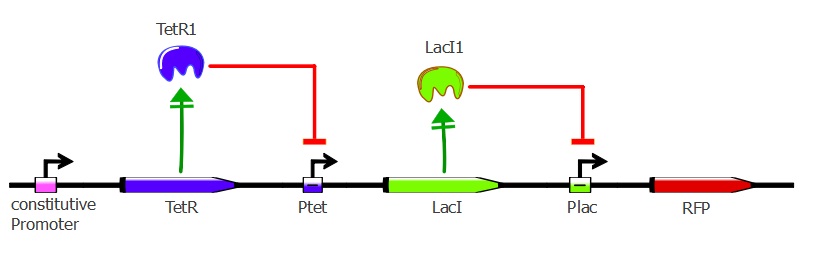
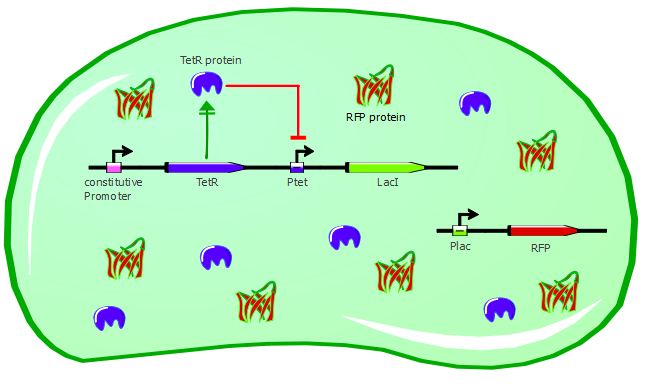
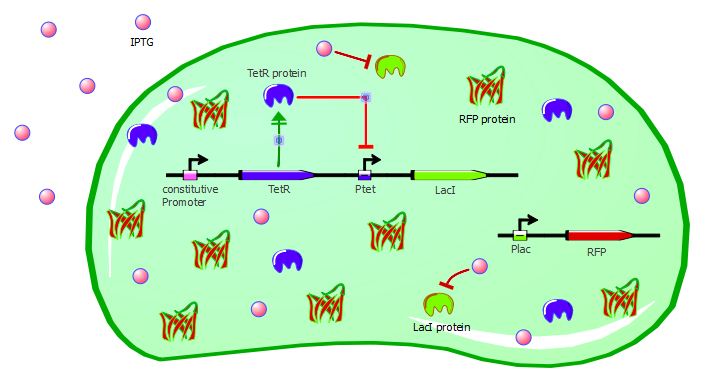
Our second system contains LacI in addition to TetR and RFP. TetR is still induced by a constitutive promoter, then LacI is under Ptet regulation and RFP is under Plac regulation. LacI plays the role of an inverter, so that we can measure directly TetR-Ptet interactions: if TetR binds to Ptet, LacI is not expressed, so RFP is not repressed and is instead expressed. Here, TetR mutants with a higher binding affinity for Ptet will result in higher levels of RFP expression.
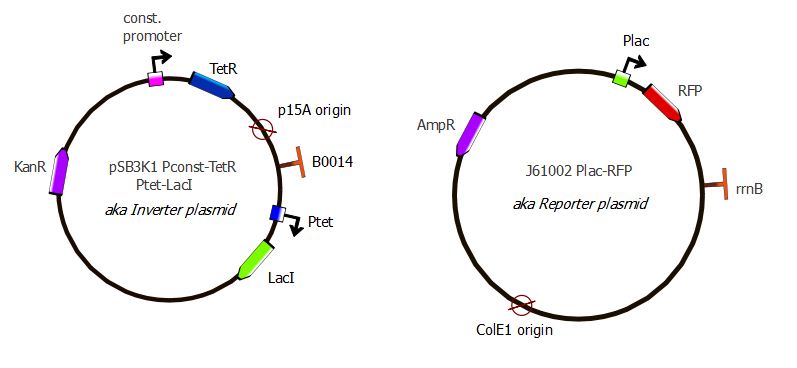
Here also we have a two-plasmid system; TetR and LacI are placed on the pSB3K1 Pconst-TetR Ptet-LacI plasmid whereas RFP is on the J61002 Plac-RFP plasmid. You can read more about these plasmids on the plasmids details page.
Experimental validation - with wild-type TetR
Our second readout system was based on TetR and LacI, where we repressed both sequentially with ATC and IPTG, respectively.
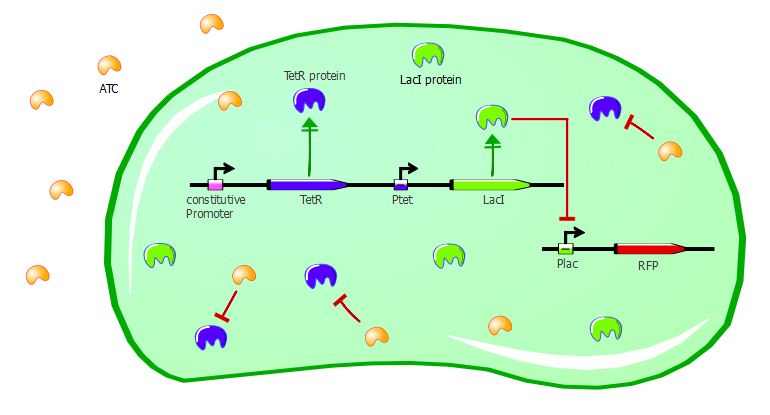
ATC induction
Adding ATC in the medium of the co-transformed cells will inactivate TetR. As a consequence, LacI will be expressed and RFP will be repressed. We should then see a decrease of RFUs correlating with increasing concentrations of ATC.
Gene expression in the cells without ATC:
Gene expression in the cells with ATC:
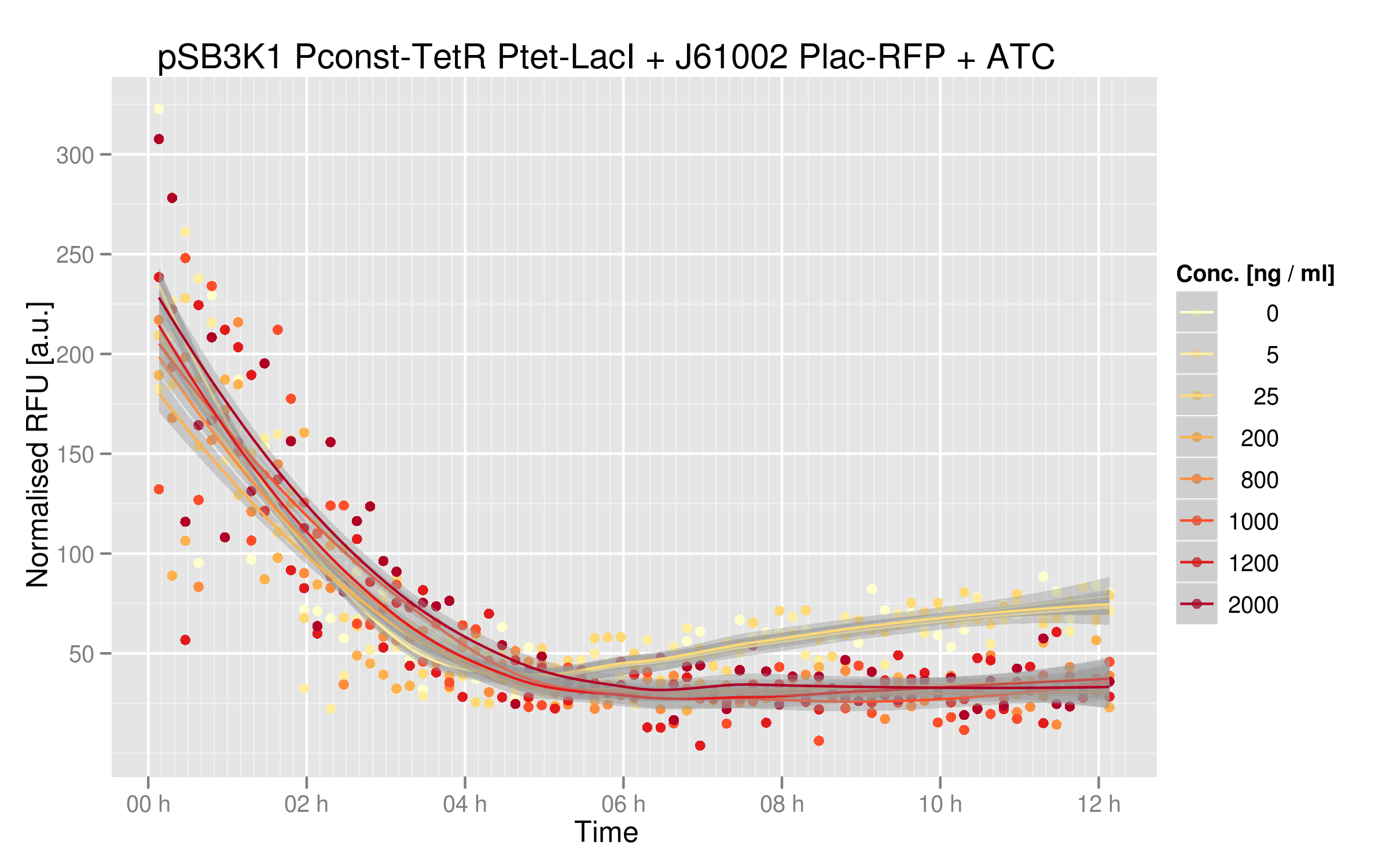
Below you can see the induction curves at different ATC concentrations added to the growth medium:
With zero or low concentrations of IPTG in the cells' medium, RFP expression is quite weak; this can be explained by the fact that Ptet was mutated in our plasmids and thus TetR couldn't repress LacI very efficiently. In normal conditions, we should see RFP expression when TetR binds to Ptet - which is the case here, since we have the wild-type TetR gene. Here, LacI was not well repressed by TetR, thus RFP is repressed even when TetR binds to Ptet. Nevertheless, there is a decrease in RFP expression when we add sufficient amounts of ATC. Even in our mutated system, TetR interaction with Ptet still has an effect on the output. There is a 2-fold difference between high ATC concentrations and zero ATC; we believe that by restoring the Ptet sequence, this difference would be higher.
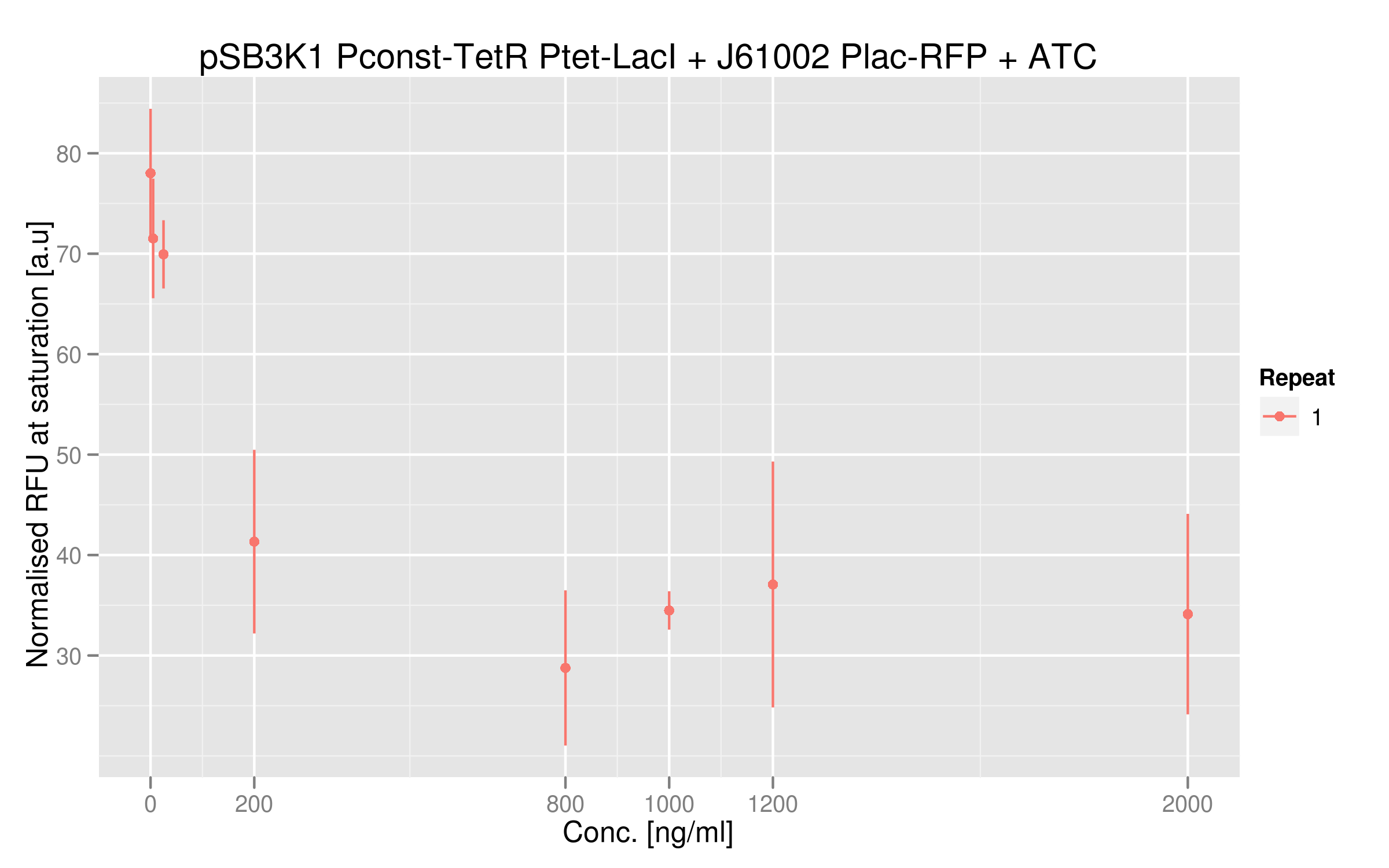
ATC dose-response curve
This data shows more clearly the difference of normalized RFUs for low or high concentrations of ATC - even if the intensities are low. As in our first readout system, the highest ATC efficiency seems to be reached with 200 ng/microL already.
IPTG induction
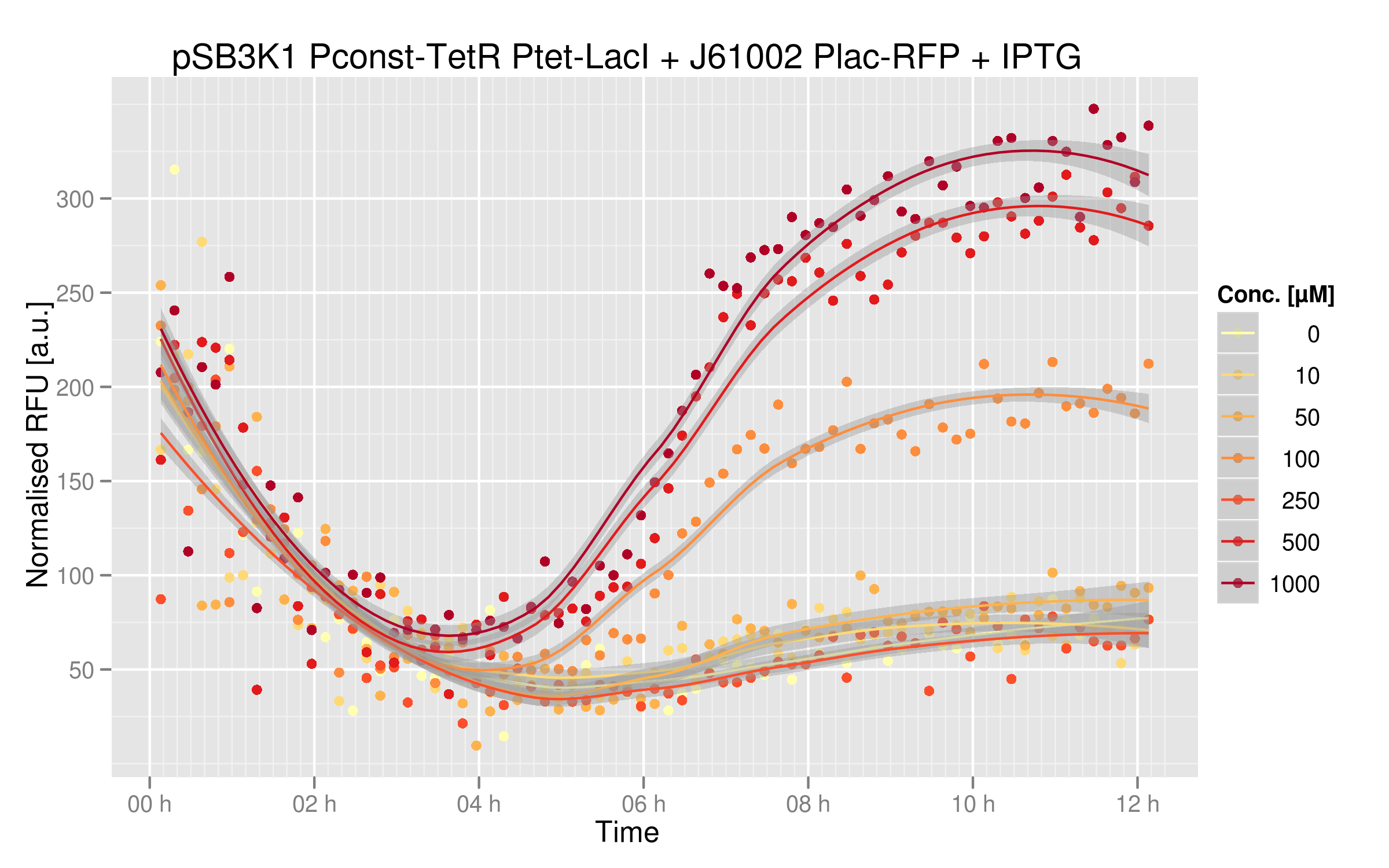
IPTG will inactivate LacI, so that RFP will be more highly expressed. From this we expect an increase in RFP expression with increasing IPTG concentration in the medium.
Gene expression in the cell without IPTG:

Gene expression in the cell with IPTG:
Below you can see the induction curves with different IPTG concentrations added in the cell's medium:
Indeed, RFUs increase 8-fold between zero IPTG and the highest concentrations. Note that the RFU intensity for the curve with no IPTG matches the intensity of the curve with no ATC in the ATC induction experiment, showing that these two experiments are consistent. The curves with the highest IPTG concentrations are not overlapping, showing that we could repress LacI even further at higher IPTG concentrations.
You can compare these results with the characterization of Plac alone. Without IPTG, RFP expression is much lower than the real Plac strength; our system does react strongly to LacI expression.
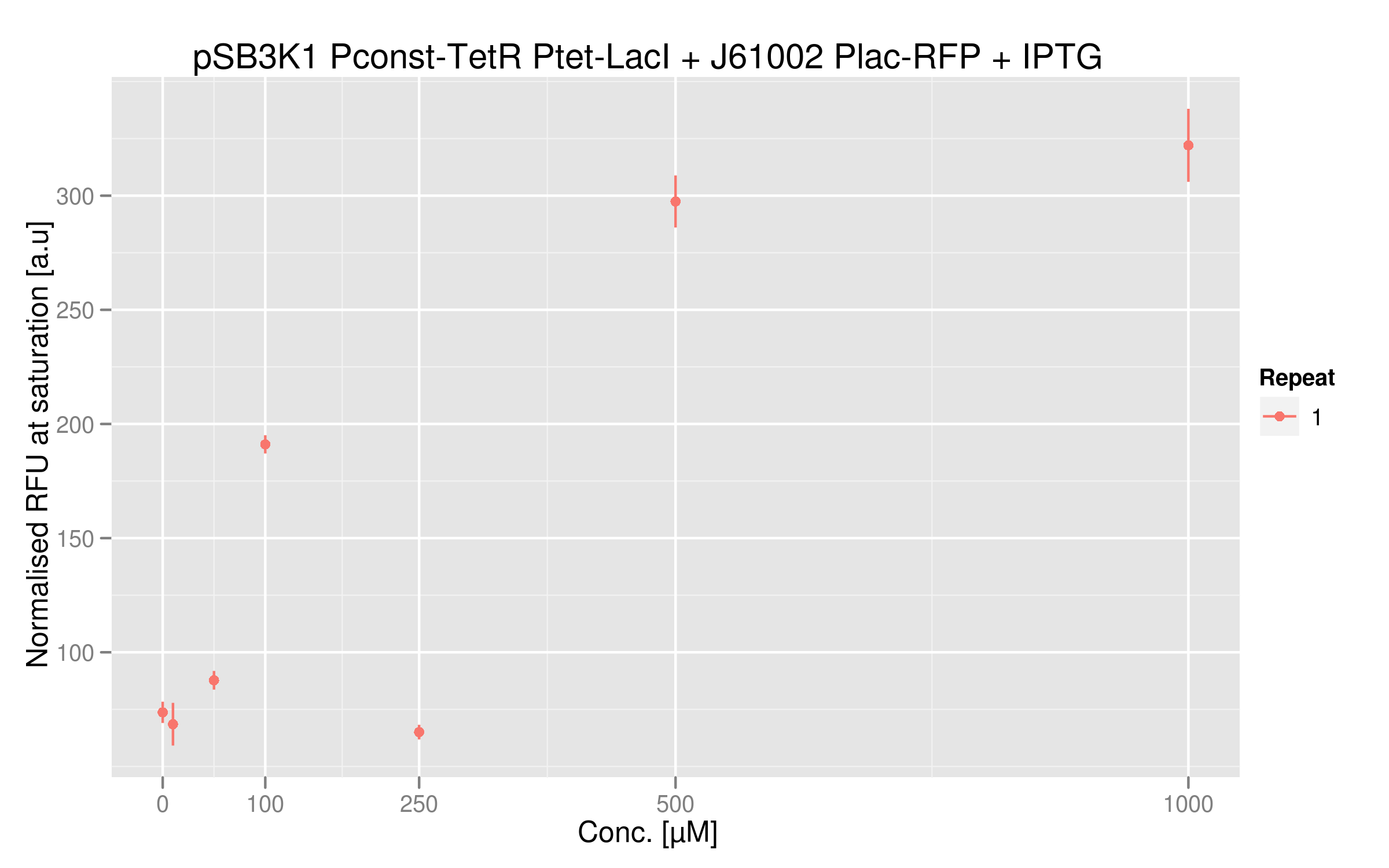
IPTG dose-response curve
Even if the RFU range is small, there is a clear parallel between RFUs intensities and IPTG dosage. An 8-fold difference between the two extremes can be sufficient for an effective and reliable readout system; however we could use a stronger Plac promoter to get more RFP expression and perhaps a bigger difference.
 "
"