Plan
The genes for this pathway were taken from the genome of arabidopsis thaliana. They were codon optimized for yeast, and had the restriction sites from the biobrick prefix and suffix removed from them to make them biobrick compatible. These in silico sequences were then constructed denovo using templateless PCR and overlap extension.
The plan for the construction of the Vitamin C synthesis pathway in yeast consists of three steps.
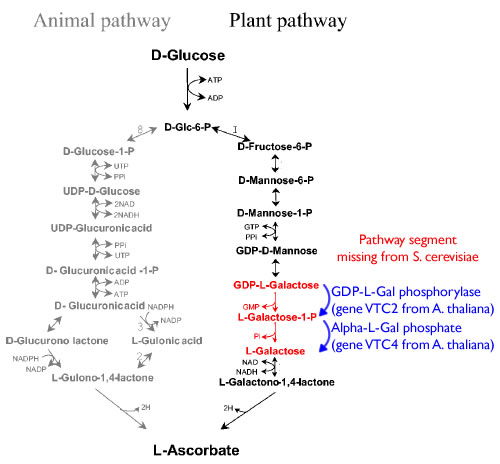
First we are going to synthesis three open reading frames corresponding to the three genes for producing GDP-mannose-3,5-epimerase, GDP-L-galactose phosphorylase, L-Galactose 1-phosphate phosphatase. This will be done using overlap extension to create two or more ‘building blocks’, each corresponding to a chunk of the orf. Each building block has a 40 base pair overlap with its adjacent building block and with the vector (pRS416) in to which we will insert the orf.
Second, these building blocks will then be assembled into the complete orf in the vector using the CPEC assembly method. The orfs will subsequently be sequenced to make sure we have constructed the correct sequences and then bio bricked using primers with the prefix and suffix flanking an overlap with the orf.
Third, these completed orfs will the be assembled into expression cassettes using under the influence of a strong promoter (list them) and terminator combination again using the CPEC method of assembly.
We will then characterize our system by growing liquid cultures and assaying samples for ascorbic acid at incremental time intervals establish rate constants as well as quantify how much vitamin C (ascorbic acid) our system can produce.
Once we have Vitamin C over time curves we will run a comparative study on how yeast producing Vitamin C grows on YPD plates (a lab standard) versus how it grows on our desired substrate, bread. To test its growth on bread we have created plates of dough like media.
To optimize the production of ascorbic acid on the new substrate we will apply a strategy of directed evolution. We will make a combinatorial library of expression cassettes using golden gate assembly for every synthesized orf in the Vitamin C pathway. This will be made using promoters and terminators of varying strengths from across the genome. Once constructed, this library will be plated on dough media plate with a calculated amount of peroxide in the agar. The presence of the peroxide will apply oxidative stress to the cells and as Vitamin C confers oxidative stress resistance this behaves as a selection for the cells that producing more Vitamin C. By adjusting the amount of peroxide on the plate we can create a threshold of ascorbic acid production below which all the cells will die. To establish which one of the library members that passed through the selection is the best, we will perform a quantitative screen on the survivors. This will be done by growing up the cells in liquid culture and then extracting the ascorbic acid and spectrophotomertically determining its concentration.
Pathway
Sauer, M., Branduardi, P., Valli, M., & Porro, D. (2004). Production of L-ascorbic acid by metabolically engineered Saccharomyces cerevisiae and Zygosaccharomyces bailii. Applied and environmental microbiology, 70(10), 6086-91. doi: 10.1128/AEM.70.10.6086-6091.2004.
Experiments
Vitamin C concentration Assay (By Anne Marie Helmenstine, Ph.D) 1. Add 25.00 ml of vitamin C standard solution to a 125 ml Erlenmeyer flask. 2. Add 10 drops of 1% starch solution. 3. Rinse your buret with a small volume of the iodine solution and then fill it. Record the initial volume. 4. Titrate the solution until the endpoint is reached. This will be when you see the first sign of blue color that persists after 20 seconds of swirling the solution. 5. Record the final volume of iodine solution. The volume that was required is the starting volume minus the final volume. 6. Repeat the titration at least twice more. The results should agree within 0.1 ml. 7. You titrate samples exactly the same as you did your standard. Record the initial and final volume of iodine solution required to produce the color change at the endpoint.
 "
"