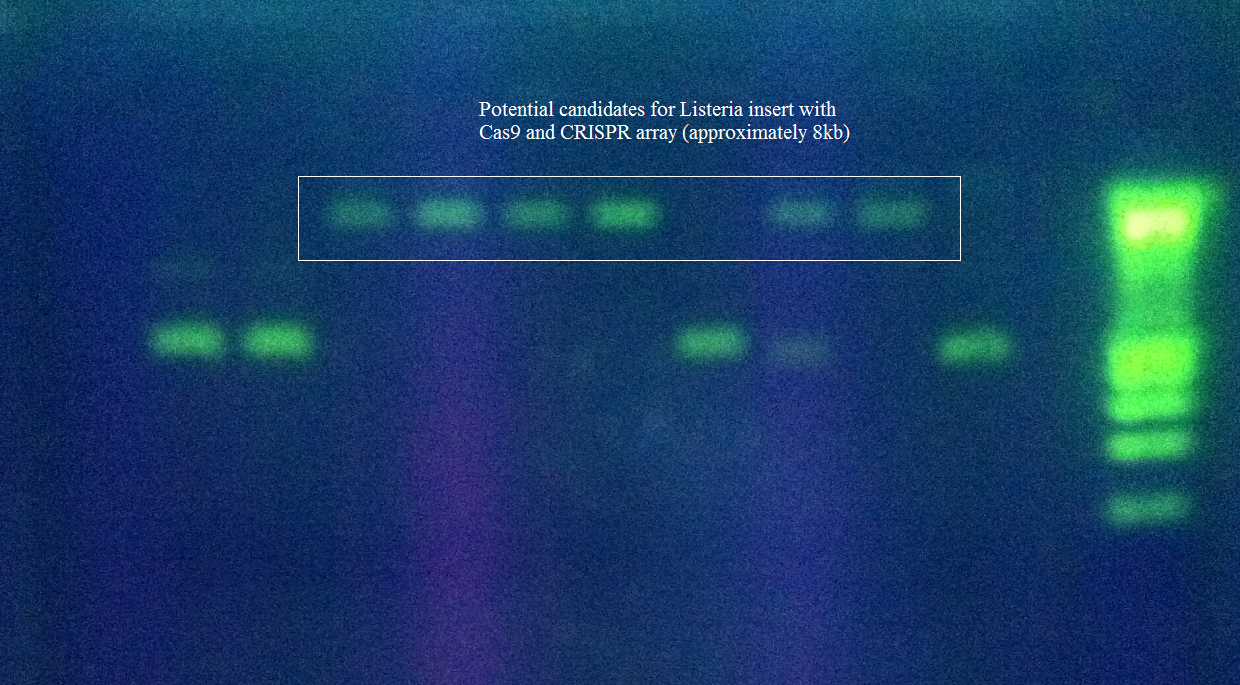
Team:Arizona State/Results/Data
From 2011.igem.org
|
|
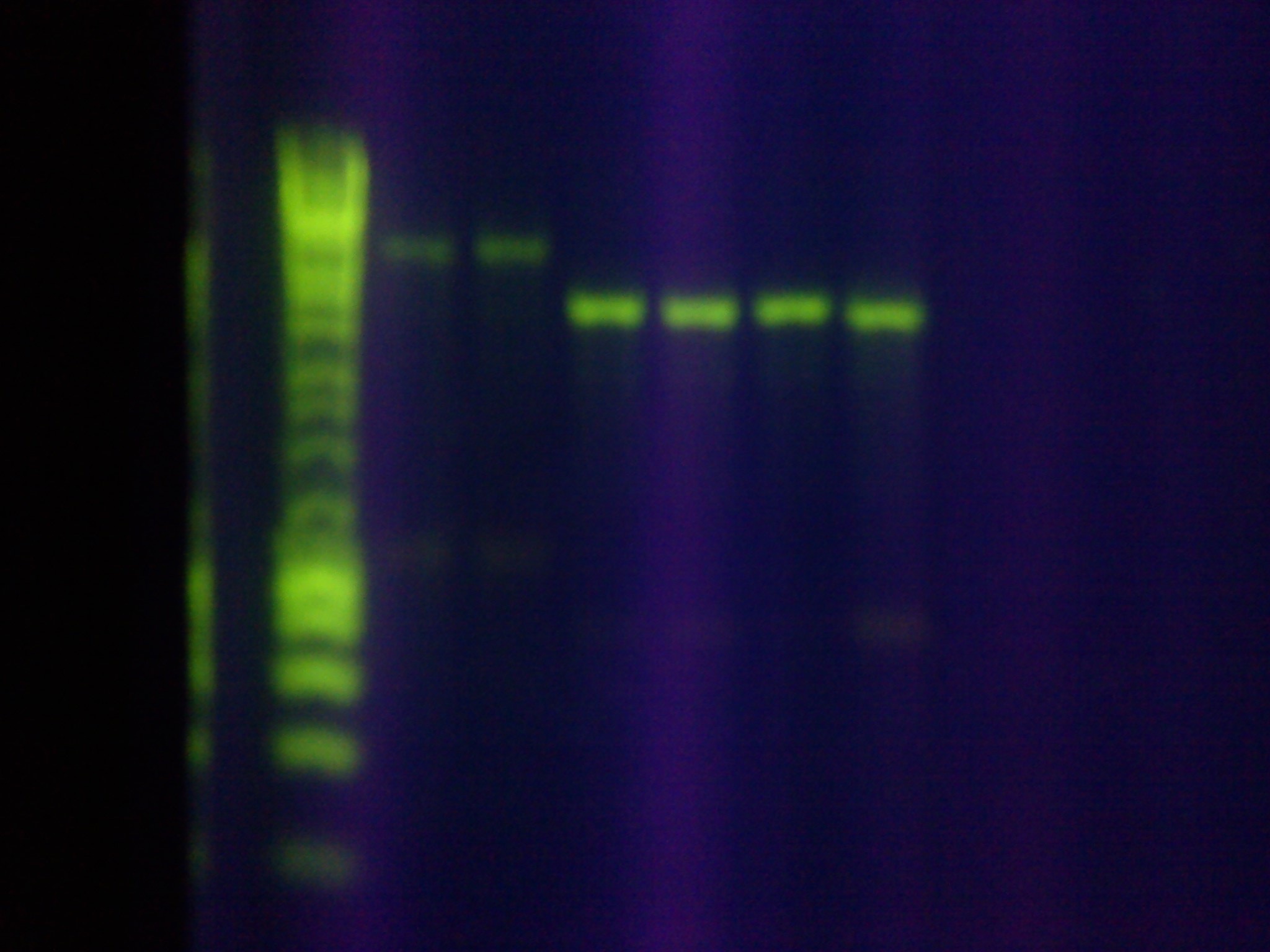
E. coliConstruction of RSR+Leader Array
Isolation of Cas genes
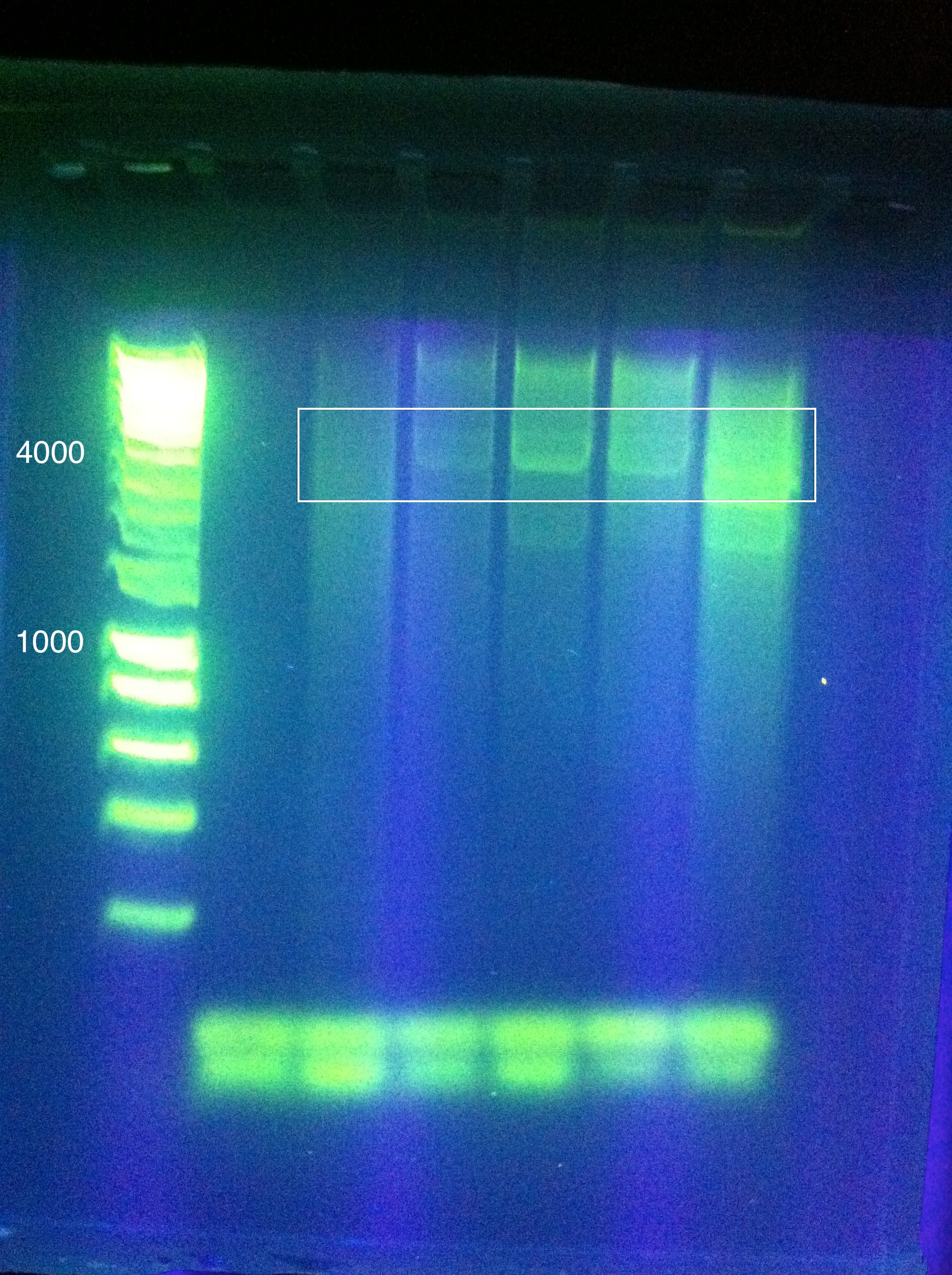
Assembly of Leader+RSR into pRSF Duet
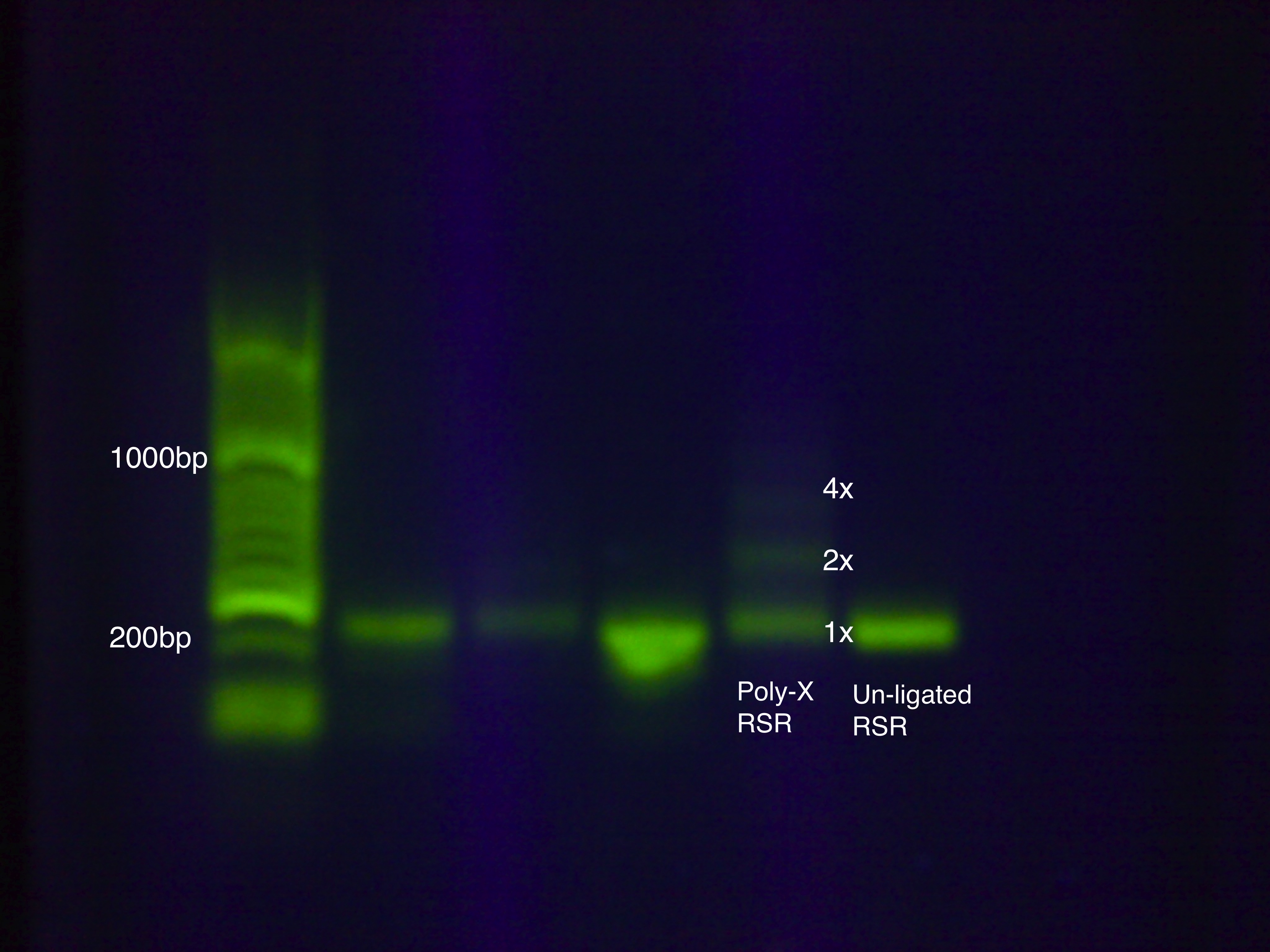
Poly-X LigationWe devised a new assembly method, the Poly-X Ligation, for RSR construction and tested it on an RSR construct with GFP. Contingency Test
*Recorded OD600 = raw absorbance * 3.2
**Weighted Colony Count = (# of Colonies)/(mean OD600 of competency prep) B. haloduransConstruction of RSR Array
Isolation of cmr genes
Assembly into pRSF DuetL. innocuaConstruction of RSR ArrayCas gene
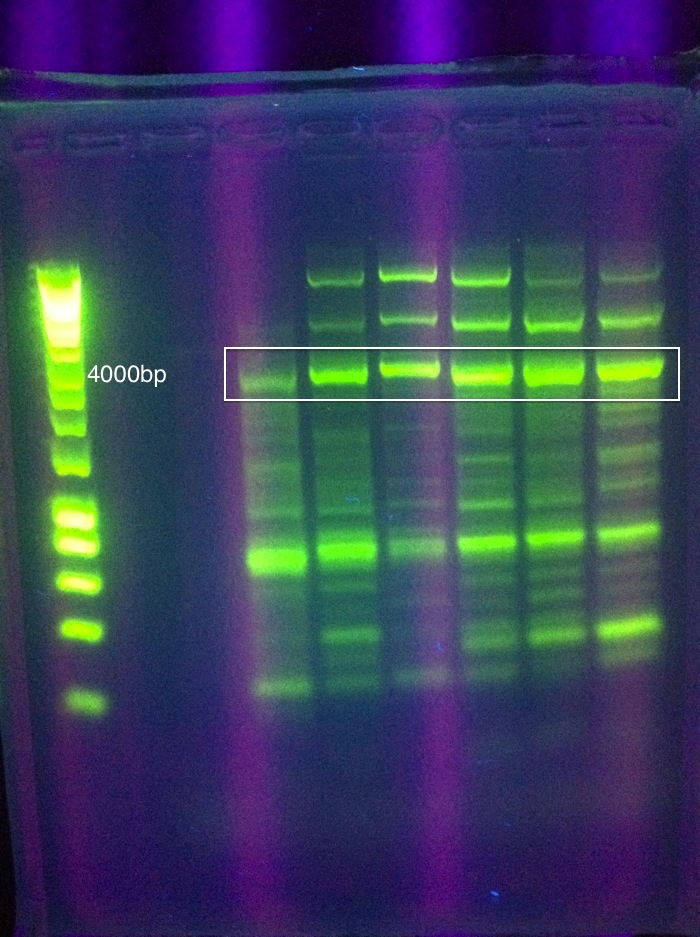
Assembly into pRSF Duet
Other
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"