Team:UNICAMP-EMSE Brazil/Results
From 2011.igem.org

| Home | Project | Methods | Results | Data | Team | Notebook | Human Practices | Safety | Profile | Sponsors | Wix |

Overview
Functional tests
The students assembled the devices subparts: the SoxR/SoxS sensor (Device 2, NO sensor device/ IL-10 producer), the Adrenaline sensor (Device 1, CA/AI-3 sensor device/ IL-12 producer), and the hemolysin secretion system (Device 3, Protein Secretion System). All the sensors were also built with GFP downstream of the sensor itself, replacing the Device products: IL-12 at Device 1 and IL-10 at Device 2.
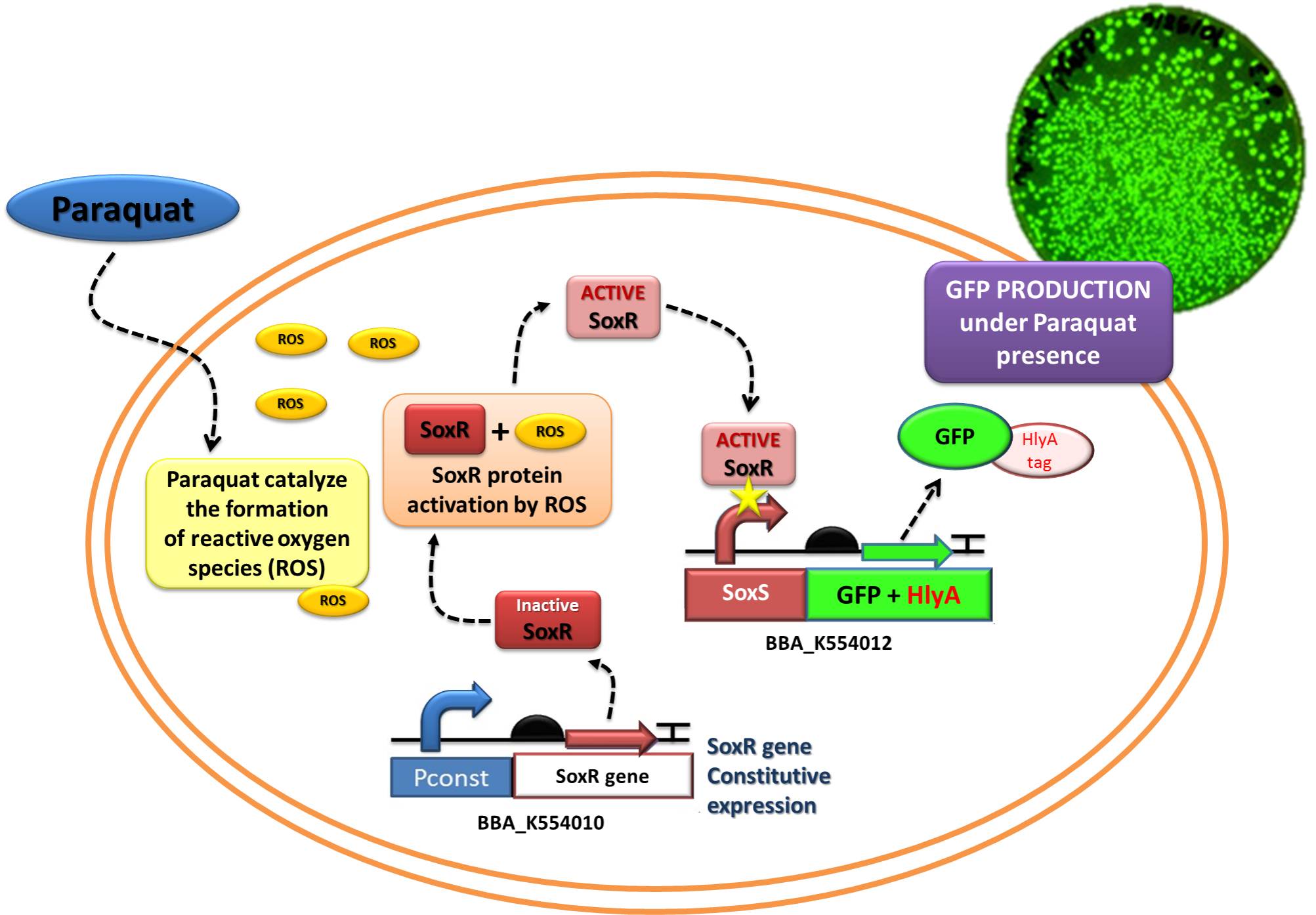
Device 2 testing: SoxR/SoxS system regulating GFP production
The Device 2 is a modification of Stanford team anti-inflammatory device (iGEM 2009 - https://2009.igem.org/Team:Stanford/ProjectPage), which comprises SoxR gene (BBa_K223047) under control of a Constitutive Promoter (BBa_J23119) and SoxS promoter (BBa_K223001 – deleted part), coupled to human IL-10 gene (sequence designed by the team, improved for bacterial expression).
In order to test the ability of Jedi Bacteria in sensing NO levels and activating genes linked to SoxS promoter, we built a Device testing, with GFP linked to SoxS promoter, as it is shown in the following schema (Figure 1):
Figure 1: Testing Device 2 through replacement of IL-10 to GFP.
The ability to recognize NO (nitric oxide), an inflammation signal molecule, was characterized for SoxR/SoxS sensor, and found to be FUNCTIONAL. In the section below we present the detailed results.

 "
"