Team:Harvard/Project
From 2011.igem.org
Bioinformatics | Chip Synthesis | Plasmid Construction | Selection Strain Engineering | Selection Results
Project Description
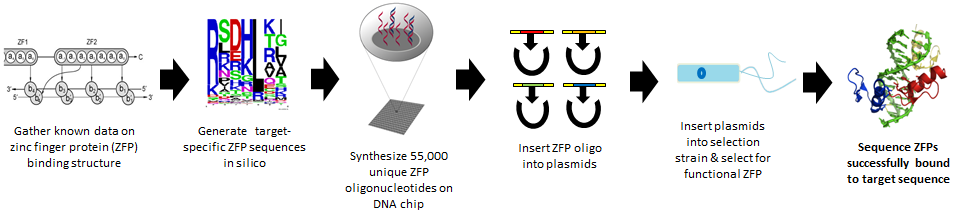
Gene therapy is a powerful approach for the treatment of disease, and holds great potential for success. While gene therapy has progressed significantly since its conception, much of its projected potential remains untapped, while existing therapeutic advancements are still fraught with serious problems. Many of these roadblocks to existing therapies are caused by non-specific gene insertion (as through viral vectors), which hinders therapeutic viability and can lead to unintended side effects including cancer. In recent attempts to overcome this obstacle, however, one particular approach has shown much promise. This approach utilizes engineered zinc finger proteins, which have been shown to edit the genome with dramatically increased accuracy (Perez et al 2008, Li et al 2011). Zinc finger proteins are naturally-occurring protein domains composed of multiple individual zinc finger subdomains. Although the individual subdomains (“fingers”) occur in a variety of structural motifs, the classic Cys2His2 (C2H2) motif is among the best characterized and can be observed in a variety of transcription factors. The C2H2 motif (see Fig. 1) consists of a beta-sheet conjugated to an alpha helix, and it is structurally coordinated through interactions between two cysteine and two histidine residues with a single zinc ion. Many eukaryotic transcription factors utilize C2H2 zinc finger domains, which possess a unique capacity to bind to specific DNA sequences. Zinc finger proteins have received increasing attention in recent research for their DNA-binding ability and specificity, which offers a solution to non-specificity in gene therapy and could thus bring the scientific community a step closer to realizing many novel therapeutic applications. Recent studies, for instance have demonstrated that DNA-binding zinc fingers coupled with Type IIs restriction endonucleases such as Fok1 can be harnessed as effective tools to genetically treat hemophilia and confer HIV resistance in mouse models (Perez et al 2008, Li et al 2011).
While many different natural zinc finger proteins can be employed for synthetic biology-based tasks, engineered zinc finger proteins are most commonly composed of an array of three zinc fingers based on Zif268. Zif268 is a well-characterized mammalian early response transcription factor also known as EGR-1 that was discovered in mice. Zif268 has three C2H2 finger subunits, and each finger binds a specific 3-nucleotide base pair (bp) triplet on a DNA strand. Composed of an array of three fingers, Zif268 binds to three consecutive DNA triplets that in total comprise a 9-bp binding site. This 3-finger domain, 9-bp specificity is a commonly occurring theme in zinc finger literature, although it is by no means a rule. Natural and engineered zinc finger arrays may contain fewer or more zinc fingers and bind DNA sequences of corresponding length and specificity. While any given zinc finger might bind a certain 3-bp DNA sequence with exquisite specificity, even the most specific 3-bp binding event is of little practical value for gene therapy, as a given 3-bp sequence may appear countless times within a given genome. Thus, for practical use in drug delivery or gene therapy, zinc fingers must be engineered to bind more specifically to DNA sequences. Such increased specificity can be achieved through the creation of multi-finger arrays that bind to specific 9-bp sequences. With this in mind, research has focused on creating modular zinc finger subunits, which can be combined into multi-finger arrays. Three-finger arrays, for instance, are tailored to bind to a specific 9-bp sequence through the selection of appropriate zinc fingers to bind to each DNA triplet.
Different studies have suggested different models of binding and varying levels of success for zinc finger modularity. Thus, through an approach utilizing data-mining, binding models, and comprehensive bioinformatics, in conjunction with next-generation oligo synthesis technology, we hope to develop a method for engineering zinc finger proteins that can bind to any arbitrarily-selected DNA sequence. The success of such an engineering method would, in turn, facilitate clinical advancements by promoting highly-specific, targeted gene therapies, and would promote personalized medicine by allowing the production of zinc finger-based therapies tailored to unique genomic sequences.
References
- Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS, Malani N, Anguela XM, Sharma R, Ivanciu L, Murphy SL, Finn JD, Khazi FR, Zhou S, Paschon DE, Rebar EJ, Bushman FD, Gregory PD, Holmes MC, High KA (2011). In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature, doi: 10.1038/nature10177 [Epub ahead of print].
- Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, Guschin DY, Rupniewski I, Waite AJ, Carpenito C, Carroll RG, Orange JS, Urnov FD, Rebar EJ, Ando D, Gregory PD, Riley JL, Holmes MC, June CH (2008). Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nature Biotechnology, 26(7), 808-16.
Clinical Applications
Colorblindness (Red Opsin)
Goal: Produce functional red opsin photoreceptor proteins in the eye
Method: Insertion of functional red opsin gene (OPN1LW [http://genome.ucsc.edu/cgi-bin/hgGene?hgg_gene=uc004fjz.3&hgg_prot=P04000&hgg_chrom=chrX&hgg_start=153409724&hgg_end=153424505&hgg_type=knownGene&db=hg19&hgsid=206379197 1]) upstream of normal locus in patient lacking the gene
Journal Articles
- http://www.nature.com/nature/journal/v461/n7265/abs/nature08401.html
- http://www.nejm.org/doi/full/10.1056/NEJMc0903652
In the News
- http://www.nature.com/news/2009/090916/full/news.2009.921.html
- http://www.scientificamerican.com/podcast/episode.cfm?id=gene-therapy-cures-colorblind-monke-09-09-16
- http://www.msnbc.msn.com/id/32879284/ns/health-health_care/t/gene-therapy-fixes-color-blindness-monkeys/
- http://www.wired.com/wiredscience/2009/09/colortherapy/
Inherited High Cholesterol (Familial Hypercholesterolemia)
Goal: Produce functional LDLR protein to remove LDL cholesterol from the blood
Method: Insertion of functional LDLR gene upstream of nonfunctional allele
- http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001429/
- http://www.genome.gov/25520184
- http://emedicine.medscape.com/article/121298-overview#a0199
Cancer (Myc Oncogene)
Goal: Knock out the oncogenic protein product and stop cancerous proliferation
Method: Targeted disruption (deletion) in mutated oncogene
- http://www.ncbi.nlm.nih.gov/gene/4609
- http://omim.org/entry/190080
Technological Applications
- The novel methods we employed in our project have the potential to revolutionize synthetic biology practices, and the way that future iGEM competitions are conducted. To learn more about the technological applications of our project, please see our Technology page
Helpful Zinc Finger Links
[http://compbio.cs.princeton.edu/zf/ Predicting DNA Recognition by C2H2 Zinc Finger Proteins]
- An SVM useful for predicting how well a given amino acid sequence will bind to a given DNA sequence
[http://www.zincfingers.org/default2.htm The Zinc Finger Consortium]
- Information & helpful resources for zinc fingers
[http://www.jounglab.org/ Joung Lab]
- Information about Dr. Joung's extensive work with zinc fingers
 "
"