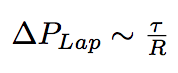Team:Paris Bettencourt/Modeling/Assisted diffusion
From 2011.igem.org

Introduction to the model
The diffusion through the nanotubes is a fast process. This speed can be partially explained by the passive diffusion through the tubes. But what if it is faster? The article from Dubey and Ben-Yehuda suggests that the diffusion is an active process. Several points can be opposed to this statement:
- First, the diffusion is happening with molecule of different natures, that have nothing to do with the natural compoments of a cell
- Unlike the mamalian cells, the tube seems not to have no "railroad" design for such a transport
The question is: can we imagine a process that is faster than passive diffusion but does not rely on specific interractions?
The answer is probably yes, and in this page we propose a new model, really challenging for the mind, but that can play a role in the diffusion process through the nanotubes.
General physical concepts and hypothesis
How a connection between two bacteria might be established
For different reasons the inner pressure of a bacterium can grow locally. That can cause the tube growth (if the pressure is big enough). If two bacteria are close to each other, their tubes could meet and even touch each other on the extremities. In this situation the phospholipids on the ends of the tubes are rather instable. Their hydrophobic part is more than even exposed to water, because of the high curvature on the extremities. So the energy preferable state is to fuse. In this case there is no specific protein involved to do the fusion, so it seems to be the closest to reality explanation of the process.
In further paragraphes we will be more interested in what happens after the membrane fusion.
Starting with a physical analogy
Imagine two bottles of gaz connected by a tube. The fist one have an higher pressure than the second one. In the first one, diluted in the gaz, there are a few molecules of another nature. We follow the destiny of these molecules.
When you open the tape, the bottle with a higher pressure will equilibrate with the other one my moving a certain quantity of it's particles through the tube in the direction of the second bottle. These moving molecules will drag with them the component diluted in the gaz and a few of these molecules will be transported to the other bottle.
From the analogy to the biology
Of course, the cell is not a bag of liquid under pressure. The water is equilibrated at both sides of the exterior membrane. The pressure, we are dealing with, is not related to water or osmotic pressure (that is a "passive diffusion thermodynamical pressure"). There is a part of the cell we are not used to think about that undergo a certain variablilty of pressure: the phospholipid membrane!
Let's evaluate the constraints that the membrane underdo. First, as it is a Gramm positive bacteria, the external sugar envelope impose the shape of the bacteria. On the other hand, the osmotic pressure is pushing the membrane against the sugar wall. At every moment inside the membrane the number of phospholipids is fixed, but this number can fluctuate depending on the phospholipid production. So finally we can pretend that our system is evoluating through a serie quasiequilibrium states.
"Though, a phospholipid is somehow behaving as a gaz trapped in a bottle. The osmotic pressure and the sugar layer are the bottle, and the number of particle trapped is giving the pressure. This pressure is likely to fluctuate depending on the divison state of the cell."
 |
 |
When the cell "start the communication", a flow of molecules can pass from the cell that have the highest membrane tension to the other one. To pass from one cell to another, phospholipids run around the tube.
The new arrived phospholipids change the membrane tension of bacterium, and under our hypothesis of constant volume, which is approved by the sugar layer that "forbids" bacterium to grow, and as a consequence they change the internal Laplacian pressure of bacterium.
where R is the raduis of bacterium, tau is a membranian tension.
A simple analogy of clothesline can help to understand what is happening. You need to pull more your clothesline to put more clothes on it. When you pull the rope by two ends you create a tension. More this tension is more weight (pressure) you can put on the rope.
All this will lead to establish the pressure difference at the tube extremities and we will get a Poiseuil flow. Constituents diluted in water will move from one cell to another unidirectionally and faster than simply diffusing. This is a fast process that we have named after the "active" and "passive" diffusion, the "assisted diffusion"
When membranes behave like a 2D Van der Waals fluid
Trapped between the osmotic pressure and the cell wall, the phospholipids particle are moving in a double layered environement. Their motion is contricted into a two dimentional motion, following the shape of the membrane. At the scale of the phospholipid, the motion can be approximated to a motion in 2D. Using statistical physics approach, we can say that the phase space has 4N dimentions: two dimentions of impulsion and two dimentions of coordinate for each particle(N is the number of particles in the system).
Can we descripe the interraction between two phospholipids in the membrane? Well, the question is complicated, but we will demonstrate that describing properly the pholpholipid, it is reasonable to fit a Lennard-Jones potential energy to this interraction. The energy of binding is smaller and the speed of sliding of one phospholipid against another is slower than the internal vibration of the chemical bounds and the internal conformers rotation of the CH2 tails. Though, in Intermolecular Forces by Israelachvili, the author shows that a phospholipid trapped in a bilayer can be aproximated by a section of cone. The section of cones is an individual, and the others are section of cone joining the previous one. The shape of the sections are giving the shape of the global structure.
 |
 |
Speaking in term of energy, once we have these cones, it is reasonable to fit a Lennard-Jones potential to this interraction. If the cones are interpenetrating, a sterical repulsion keeps the molecules appart, and the hydropatic interraction that joins one lipid with the other act as an attractive force keeping the coherence of the mombrane. We will discuss later about the value of the two coefficients.
Is this phenomenon important? Back-of-the-envelope calculation
Let's start with a rough estimation of the flux transmitted through a nanotube. Considering that 5% of the surface area of one bacterium could be transmitted through the nanotube to a second bacterium. An order of magnitude of the transmitted surface is 10-14. A nanotube of the raduis 100 nm (that is the mean value of raduis taken from the article) with this surface would be 0.5 µm long. The nanotubes observed in Dubey and Ben-Yehuda experiments were about 100 nm to 1 µm.
Let's take for example a tube 0.2 µm long. That means that 2.5 tube units will be used in the process. We can imagine it splited in two stages: the first is the establishment of a contact between two bacteria, for that 1 tube unit will be used. The rest 1.5 tube units will be used to transport the liquid inside the tube. As the diameter of the tube is of order of 100 nm, we can neglect the complexity of the fluid movement inside. In our model we will consider the fluid moving with the membrane. So the volume of transmitted liquid is about 10-20 m3, whereas the cell volume is 5*10-18 m3.
We consider then that some molecule is produced in the first cell and it's uniformally distributed. Then producing 10'000 molecules in the first cell, we can expect about 200 molecules transmitted through one nanotube to another cell. That number of molecules seems to be sufficient to be detected.
The membrane tension calculation
Considering one membrane a sphere of the radius R, on which the phospholipid double layer is uniformally distributed, we can find approximately a part of the hole sphere surface per one phospholipid :
The caracteristic distance between two neighbor phospholipids on the membrane can be written like this :
where N is the number of phospholipids on the membrane.
From this moment on we will work in canonical ensemble. The temperature is fixed and so is the number of particles in the system N. We also consider that the phospholipids can move only on the sphere surface that means that we will study the evolution of our system in 2N dimentional phase space.
The partition function will look like :
where the Hamiltonian is :
Knowing the partition function is a great deal. We can derive a free energy
of each sphere, total free energy and the membranian tenstion from it :
For two spheres we get :
and
Back to the classical physics
Now that we can calculate the tension of the membrane (i.e. the pressure of the Wan der Waals fluid), we can start calculating the equential equation of the relaxation of the membrane. As it is a fluid with an intrinsic viscosity, we will use the Navier-Stokes equation.
Let's first choose a point of view of considering the flux. There is the Bernouilli point of view in which we track the fluid particule destiny and the stokes point of view in which we are fixed in one point and watch the flux of fluid particle. The second point of view is easier for us, because, we want to count in the end the a certain quantity of phopholipids that have passed through the tubes, and link that to the quantity of water.
Let's imagine that we are an observer sitting at the entrance of the nanotube. He watch the liquid passing between his legs, and canlculate the flux.
... ...
Your job agains Oleg, the games get it's tracks!!
... ...
Finally with a difference of pressure P2-P1, we can estimate the caracteristical time of the relaxation to be:
where...
Getting back to the quantity of water
We are in the situation where a tube, that have good hydrophilic properties is moving, the tube is small and the liquid viscuous (the density of the cytoplasm over the tensity of water is about 3.2). Water will be dragged in the tube.The fluid will undergo a Poiseuille flow, but this time the tube is moving and the water following.
... ...
Going down the Poiseuille equation you will estimate the total flux dragged for a certain quantity of phospholipids that have passed bhy depending on the size of the tube, the viscosity of water...
... ...
Getting the good parameters
It will take some time, but once you have the equation, from the size of a vesible in equillibium, when we know the number of phospholipids. The viscosity has probably been estimated by FRAP techniques. For the rest, we will find out.
Analysing the results
Can this process be an efficient way for passing molecules??
Conclusion
 "
"