Team:Washington/Celiacs/Methods
From 2011.igem.org
Contents |
Redesigning Kumamolisin to Have Higer Activity at Low pH
Using Foldit to Produce Mutations
In order to create mutations from wild-type kumamolisin, we first loaded the enzyme structure into the protein editing game Foldit. Foldit allows the user to modify the way in which a protein folds by changing the amino acid sequence. Using Foldit, we modified the amino acid residues around the active site of kumamolisin which contained within it a Proline-Glutamine-Lysine-Proline (PQLP) motif found throughout the gliadin peptide of gluten. With the PQLP locked in place along with the key active site amino acid residues, mutations were made which appeared to stabilize PQLP within the active site. The purpose of stabilizing the PQLP motif within the active site was to both increase the activity of the enzyme and to increase specificity towards the PQLP amino acid sequence. Using this method, we produced several mutants at a time which could potentially increase the enzyme's proteolytic activity towards gliadin.
Mutagenizing Kumamolisin
Producing ssDNA
Once we had obtained feasible mutants from the Foldit program, we ordered these mutations in oligonucleotide primers to be used in Kunkel mutagenesis. To perform the mutagenesis, we first transformed the plaasmid containing wild-type kumamolisin into chemically competent cells of Escherichia coli CJ236. These cells were then grown and harvested for their single stranded DNA (ssDNA).
Kunkel Mutagenesis
Using ssDNA as a template, we annealed oligo nucleotides in a PCR block to increase specific binding. The primer was then extended on the ssDNA using T7 DNA polymerase. We then dialyzed the DNA to remove salts introduced in previous steps. We then added the DNA to cuvettes containing electrically competent E. coli BL21* cells. These cells were then pulsed with electricity to allow the uptake of the newly mutagenized DNA. After growing the cells for an hour in TB, we plated the cells on LB agar containing kanamycin.
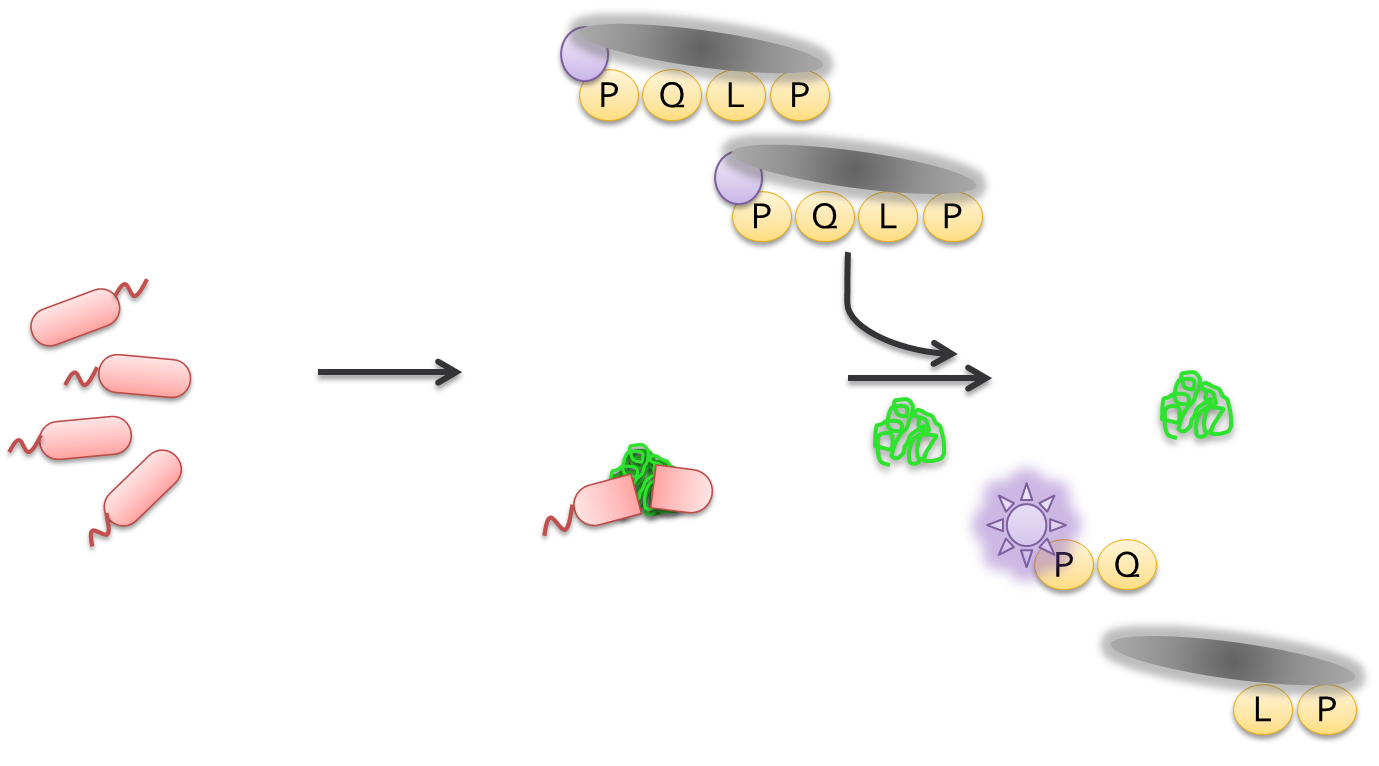
Using a Whole Cell Lysate Assay to Test Feasability of Mutants
After the cells had been allowed to grow overnight, colonies were picked and used to inoculate a 96 well plate containing LB and kanamycin. This step allowed us to grow a representative amount of cells containing each mutation. After growing overnight at 37 degrees celcius cells from each well were transferred to another 96 well plate containing TB and kanamycin. These plates were incubated at 37 degrees celcius and later induced using IPTG. After induction, we incubated the plates at 18 degrees celcius overnight. We then lysed the cells and tested the supernatant for proteolytic activity towards PQLP in an assay which measured PQLP degradation over a period of 30 minutes. The assay was done at pH 4 in accordance with the assays done to test ScPEP according to the literature. The mutants were tested against wild-type kumamolisin and ScPEP, an enzyme currently used for the treatment of gluten intolerance via proteolysis. The assay we used was not highly accurate in terms of actual activity. However, what the assay allowed us to do was determine activity relative to our controls. This allowed us to determine which mutants were worth purifying to get more accurate activity data.
Testing Purified Mutants to Accurately Assess Activity
Purification
After compiling a set of mutants which showed a relative increase in activity we proceeded to purify our mutant proteins. This step is crucial because it allows us to determine how our mutant compares with the wild-type on a quantitative level. For instance, if the whole cell lysate assay showed one mutant to have ten times the activity level as wild-type kumamolisin we cannot assume that there has been an increase in activity because there could simply be ten times the amount of protein with the same level of activity. To purify our mutants, we first grew them in TB and kanamycin with a single colony of the mutant. This inoculation was grown over 24 hours at 37 degrees celcius and then expanded to 50 mililiters (TB+kanamycin). We then allowed the culture to grow to a specific optical density of cells before inducing the culture using IPTG. We then allowed the cells to grow again to express kumamolisin. We then lysed the cells using a lysis buffer and centrifugation. This allowed the enzymes to be released from the cells. The proteins were then collected on a column, washed with buffer, and eluted off the column. Finally, wwe dialyzed the protein in a sodium acetate buffer (pH 4).
Assay
Once we had pure protein, we determined the concentration of each using a NanoDrop machine. With the concentration of each, we then diluted the proteins to the same concentration. For the assay, we also used purified wild-type kumamolisin and ScPEP. The assay was again run for 30 minutes at pH 4. Using the data from the purified assays, we were able to deermine which mutants had higher activity than kumamolisin and by how much their activity was greater.
 "
"