Team:Cornell/Results
From 2011.igem.org
Rockyshell92 (Talk | contribs) (→Invitro Biosynthesis) |
Rockyshell92 (Talk | contribs) (→Invitro Biosynthesis) |
||
| Line 126: | Line 126: | ||
| - | [[file:VioResults Cornell2011.jpg| | + | [[file:VioResults Cornell2011.jpg|700px|center]] |
=Light-Induced Lysis= | =Light-Induced Lysis= | ||
Revision as of 21:31, 28 October 2011
Results |
Protocol |
Notebook |
Parts Submitted
Contents |
Results Summary
Enzymatic Pathway
Through the summer, we successfully constructed the tagged violacein pathway enzymes VioA, VioB, and VioE and submitted those parts to the registry. These three enzymes in series are capable of converting L-tryptophan into prodeoxyviolacein, a dark green colored intermediate of the violacein pathway. Each enzyme sequence was constructed to have the AviTag sequence included on its C-terminus, followed by the stop codon so that one single fusion protein is expressed. The AviTag is capable of biotinylating the enzymes so that they can be bound to surfaces using a simple NeutrAvidin ligand system.
To simplify the construction process, each enzyme sequence was PCR'd off the iGEM Distribution sample with a KpnI site followed by the iGEM prefix, the gene of interest, and ended with an SphI or HindIII site that replaced the location of the stop codon. These PCR products could then be ligated into a modified pZE12-SMCS backbone which contained the AviTag sequence followed by the stop codon, iGEM suffix and ClaI site. This backbone was constructed using the primer insert method outlined in the Project Description.
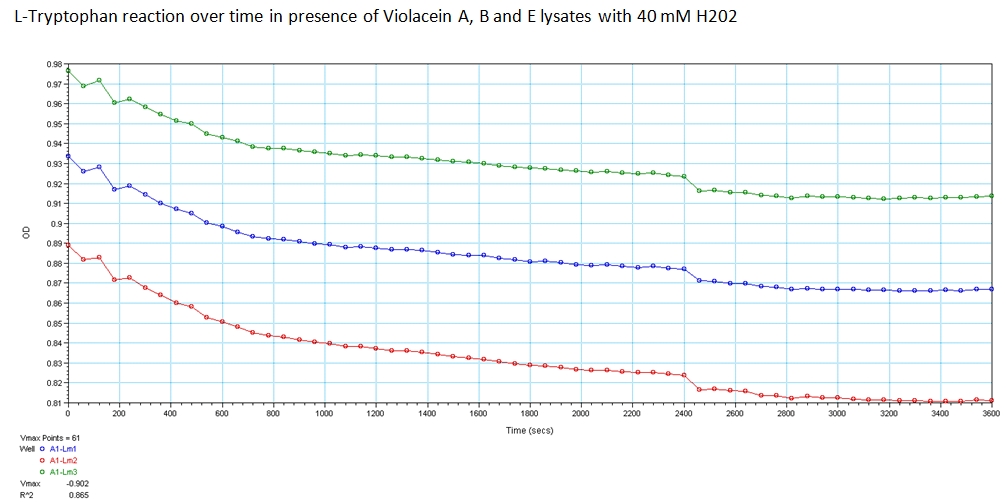
With the modified enzymes, we performed experiments to show their expression and utilization of tryptophan using a UV/Vis spectrometer at the peak wavelengths at which tryptophan responds to (590,638, and 562 nm). The OD measurements of solutions contain equivolume of VioA, VioB, 40 mM Hydrogen Peroxide (for oxidation of tryptophan) and VioE lysate with varying concentration of tryptophan. The results are shown below.
The green points represent readings at 562 nm, blue at 590 nm, and red at 638 nm at 0.008 g/mL concentration of L-Tryptophan and 40 mM H202. As you can see, the absorbance of tryptophan noticeably decreases over a time course of 1 hour. This experiment was recreated varying the ratio of enzymes and concentration of initial tryptophan. The results showed a similar decreasing trend.
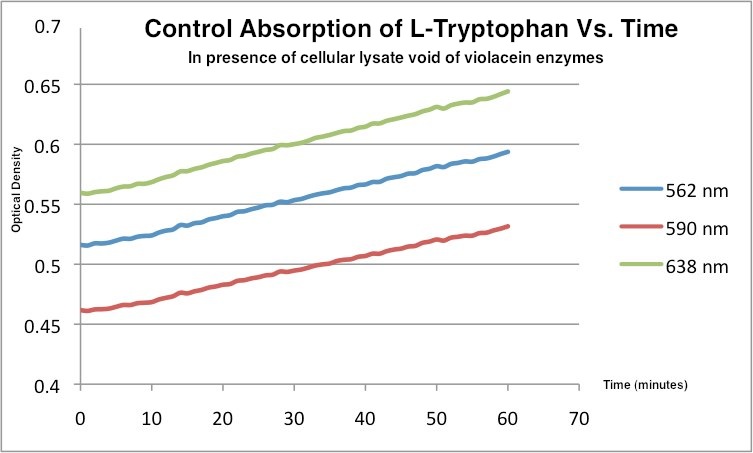
This is the control tryptophan readings. In the absence of violacein enzymes, the amount of tryptophan showed the opposite trend.
Peak wavelength values were found with the following reference
Sánchez, C Reevaluation of the violacein biosynthetic pathway and its relationship to indolocarbazole biosynthesis. Chembiochem : a European journal of chemical biology Vol. 7, 8, 2006, p. 1231
Microfluidics Assembly
Binding Experiment
Goal - to determine if the biotinylated enzymes would attach to the NeutrAvidin coated wall.
Experiment
- Negative Control - chip that is not coated with NeutrAvidin through which we flowed ATTO 590.
- Test - NeutrAvidin coated chip through which we flowed ATTO 590.
- Flow Rate - 5 µL/min
- Run Time - 20 min
- After the experiment, air was flown through the chips and pictures were taken.
Results
- The red fluorescence indicates that the enzyme successfully bound to the channel wall.
Control Test


Lysate Experiment
Goal - to determine the effect lysate would have on the binding efficiency between the NeutrAvidin coated walls and the biotinylated enzymes.
Experiment
- Positive Control - NeutrAvidin coated chip through which we flowed ATTO 520
- Negative Control - uncoated chip through which we flowed ATTO 520
- Test - NeutrAvidin coated chip through which we flowed a 5:1 ratio of lysate to ATTO 520.
- Flow Rate = 5 µL/min
- Total Run Time = 30 min
- Entire setup was covered in aluminum foil and the lights were dimmed.
After the experiment, air was flown through the chips and pictures were taken. Channels were filled with deionized water and stored in the 4 degree fridge wrapped in aluminum foil.
Results
- The test chip showed a lower level of fluorescence compared to the positive control chip. This indicates that lysate has a small inhibitory effect on the binding between NeutrAvidin and biotin.
Negative Control Positive Control Test



Continuous Flow Experiment
Goal - to determine how long the attached enzymes would stay attached under continuous flow.
- Negative Control - noncoated chip
- Test - Flow DI water through a chip with ATTO 520 attached to the NeutrAvidin coated PDMS for 15min.
- Flow Rate = 200 µL/min
- Total Run Time = 45 min (three 15 min intervals)
- After each interval, the chip was washed with air and a picture was taken in air.
Results
- The pictures indicate that the fluorescence gradually decreases under continuous flow. This suggests that the chips might have to be recoated after extended wear.
Control After 15 min After 30 min After 45 min




GFP BioBrick
Goal - to determine if our AviTagged GFP will bind to our NeutrAvidin-coated chip.
Experiment
- Negative Control - A coated chip that is flushed with deionized Water
- Test - A chip coated with NeutrAvidin with filtered GFP lysate flown through
- Flow Rate - 5 µL/min
- Total Run Time - 20 min
- The GFP containing cells were lysed with BugBuster and filtered through a PD-10 Desalting Columns to remove excess biotin. Afterwards the chip was stored in deionized water in the fridge in aluminum foil. 4 days later we took the chip out and imaged it again. Then deionized water was flushed through and the chip was reimaged. It was seen that most of the binding occurred at the inlet port and the first channel. By the second channel, no fluorescence was detected.
Results
- The presence of green fluorescence shows that our BioBrick successfully bound to the channel wall. Furthermore, green fluorescence was still present even after the chip was stored for 4 days in the fridge. However, the fluorescence after 4 days was significantly less than the initial fluorescence.
Control Initial Test (in water) After 4 Days




Invitro Biosynthesis
Goal - to determine if prodeoxyviolacein can be produced in our cell free system.
Experiment
- Negative Control - 3 coated chips (blank chips) with tryptophan flushed through
- Control 1 - VioA chip, VioB chip and a Blank chip
- Control 2 - VioA chip and Blank chip ::*Test - VioA chip, VioB chip and VioE chip
- Flow Rate - 5 µL/min
- Total Run Time - 6 hours
- Enzymes were incubated in the chips for 1 hour with no flow. The enzyme solution was
- then replace with fresh enzyme solution and incubated again for 1 hr.
Results
- z
Control Initial Test After 4 Days





Light-Induced Lysis
We designed the light-induced apoptosis system using APE and submitted the part to Invitrogen Life Technologies for synthesis. Given our final construct was in excess of 6,000 bp, synthesis is currently being performed into two subsections. The first of which is the light sensitive promoter (Pcpcg2) followed by the lysis cassette and the genes necessary for the biosynthesis of phycobilins. The second component was the CCaS and CCaR. CCaS is the surface 532 nm green light activated protein which phosphorylates, and activates, CCaR to bind to the light sensitive promoter and express the downstream gene.
So far, we have received the second component of our light system, and we have BioBricked this part for submission and use with the international parts registry. Please see [http://partsregistry.org/wiki/index.php?title=Part:BBa_K597105 this page] for more information.
 "
"