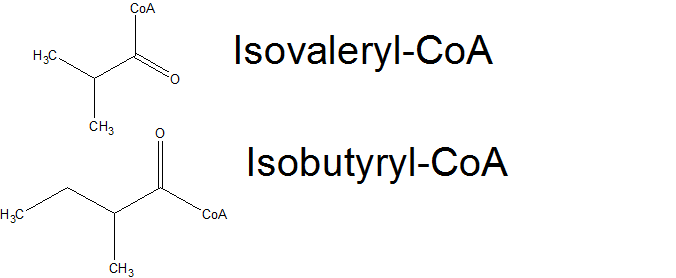Team:Washington/Alkanes/Future/FabH2
From 2011.igem.org
(→FabH2 is toxic to E. coli) |
(→Reducing the Toxicity of FabH2.) |
||
| Line 19: | Line 19: | ||
=Reducing the Toxicity of FabH2.= | =Reducing the Toxicity of FabH2.= | ||
| - | We hypothesized that if we could reduce the toxicity of FabH2, we could see the production of alternative alkane products. The original FabH2 system placed the FabH2 gene under the control of a strong constituitive promoter. We thought that if we could reduce FabH2 expression, we could decrease toxicity and be able to see the effects of FabH2 production on the alkanes produced by the PetroBrick. We cloned FabH2 into a [http://partsregistry.org/wiki/index.php?title=Part:BBa_K314103 low copy number 3k3 IPTG inducible vector]. By reducing copy number appproximetly 10 fold, and by having the ability | + | We hypothesized that if we could reduce the toxicity of FabH2, we could see the production of alternative alkane products. The original FabH2 system placed the FabH2 gene under the control of a strong constituitive promoter. We thought that if we could reduce FabH2 expression, we could decrease toxicity and be able to see the effects of FabH2 production on the alkanes produced by the PetroBrick. We cloned FabH2 into a [http://partsregistry.org/wiki/index.php?title=Part:BBa_K314103 low copy number 3k3 IPTG inducible vector]. By reducing copy number appproximetly 10 fold, and by having the ability to control expression, we thought that we could lessen toxicity and observe even chain length alkane production. We called this inducible, low expression construct the FabBrick, a modular add-on to the standard PetroBrick. |
| - | + | =FabBrick Induction Results in Even Chain Length Alkane Production= | |
| + | The FabBrick was co-transformed with the Petrobrick in XL1-Blue E. coli. cells were grown up in rich TB media+ 5uM IPTG, and innocultated to OD10 in M9 production media + 5uM IPTG. Alkanes were extracted after 24 hours. In addition, GC runs were performed on uninduced FabH2/Petrobrick cultures, and on cells expressing only the PetroBrick. | ||
| + | There was no significant difference between the GC peaks in the PetroBrick extract and in the uninduced FabH2/PetroBrick Extract. All of the peaks in these two extracts that fall within the expected elution time of the C16 alkane( 9.5-10 min) correspond to trace amounts of compounds that we cannot identify. When FabH2 is induced, we see a strong new peak at approximetly 9.75 minutes. The MS spectra of this peak is highly consistent with C16 alkane. The overall ion fragmentation fingerprint is identical to that alkane. Identification as a C16 alkane is confirmed by the presence of a strong parent ion at a mass of 226, exactly the mass of the C16 alkane. This confirms production of C16 alkane. In addition, some C14 alkane production was observed. This is the first time that even chain length alkanes have been produced recombinately, and expands the PetroBrick system to be able to produce all of the alkanes in the range C13-C17. Note that this peak corresponds to only trace levels of alkane( approximately 1mg/L), and future optimization is needed to increase even chain length production levels. | ||
==References== | ==References== | ||
1.Beta-ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. Choi KH, Heath RJ, Rock CO. J Bacteriol. 2000 Jan;182(2):365-70. | 1.Beta-ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. Choi KH, Heath RJ, Rock CO. J Bacteriol. 2000 Jan;182(2):365-70. | ||
Revision as of 20:52, 24 October 2011
Background
The basic alkane production system is incapable of making branched chain alkanes, as E. coli normally only makes straight chain fatty acids. In addition, our basic system is only able to make odd chain length alkanes, as E. coli only normally make even chain length fatty acids. We thought that if we could introduce an enzyme into PetroBrick expressing E. coli that results in the production of branched chain or odd chain length Fatty acids, we could cause the PetroBrick to produce branched chain or even change length alkanes. One protein whose expression has been previously shown ( [1]) to cause E. coli to produce branched chain and odd chain length fatty acids is FabH2 from Bacillus subtilis. The FabH family of proteins initiates fatty acid elongation by converting an Acyl-CoA into an Acyl-ACP, with is extended by 2 carbon units to form longer chain length fatty acids. Normally, FabH proteins use a simple 2-carbon acetyl-CoA to start fatty acid biosynthesis, resulting in even chain length linear fatty acids. However, FabH2 has been hypothesized to initiate fatty acid biosynthesis using Isobutyryl-CoA and Isovaleryl-CoA( products from Valine and Leucine degredation), resulting in 2-methyl branched fatty acid production, as well as using a 3-carbon propanoyl-CoA, resulting in odd chain length fatty acids. If we could use FabH2 and the Petrobrick to get E. coli to produce both alkanes and branched chain or odd chain length fatty acids, we should theoretically be able to produce 2-methyl branched alkanes or even chain length fatty acids.
Methods
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K590034 FabH2 ] was constructed from oligos( refer to protocol). FabH2 was then amplified to add an [http://partsregistry.org/wiki/index.php?title=Part:BBa_B0034 Elowitz standard RBS]. This RBS-FabH2 construct was cloned into the 3' end of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K590025 the PetroBrick] to express [http://partsregistry.org/wiki/index.php?title=Part:BBa_K590030 FabH2 as well as AAR and ADC]. In addition, we cloned fabH2 into a [http://partsregistry.org/wiki/index.php?title=Part:BBa_K314103 low copy number IPTG inducible vector].
FabH2 is toxic to E. coli
Throughout our experiments, we observed that cells expressing FabH2 grew significantly slower than any of our other alkane producing cells. In order to quantify this effect, we measured OD600 for alkane production constructs every 24 hours for a 72 hour period, producing a growth curve.
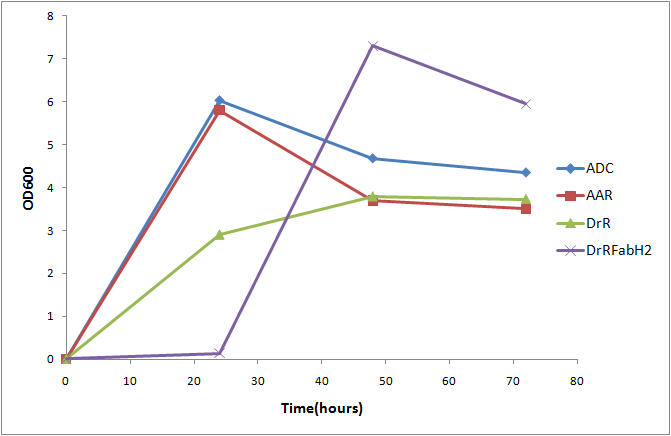
After 24 hours, FabH2 producing cells had barely grown at all, indicating a severe growth deficiency. The cells were able to rapidly grow after 24 hours, presumably due to a mutation that counteracted the negative effects of FabH2. This toxicity could be due to branched chain fatty acid production, or due to the activity of FabH2 on straight chain substrates affecting cellular metabolism. This severe growth deficiency implies that FabH2 is being expressed, and has activity that is detrimental to cell growth. Due to FabH2's toxicity, any time that we coexpressed fabH2 and the PetroBrick on a high copy number, strong promter expression vector, total alkane yield was decimated. In cells expressing the normal PetroBrick, we were able to consistently Reduction of this toxicity should allow us to determine the effects of FabH2 production on alkane biosynthesis.
Reducing the Toxicity of FabH2.
We hypothesized that if we could reduce the toxicity of FabH2, we could see the production of alternative alkane products. The original FabH2 system placed the FabH2 gene under the control of a strong constituitive promoter. We thought that if we could reduce FabH2 expression, we could decrease toxicity and be able to see the effects of FabH2 production on the alkanes produced by the PetroBrick. We cloned FabH2 into a [http://partsregistry.org/wiki/index.php?title=Part:BBa_K314103 low copy number 3k3 IPTG inducible vector]. By reducing copy number appproximetly 10 fold, and by having the ability to control expression, we thought that we could lessen toxicity and observe even chain length alkane production. We called this inducible, low expression construct the FabBrick, a modular add-on to the standard PetroBrick.
FabBrick Induction Results in Even Chain Length Alkane Production
The FabBrick was co-transformed with the Petrobrick in XL1-Blue E. coli. cells were grown up in rich TB media+ 5uM IPTG, and innocultated to OD10 in M9 production media + 5uM IPTG. Alkanes were extracted after 24 hours. In addition, GC runs were performed on uninduced FabH2/Petrobrick cultures, and on cells expressing only the PetroBrick.
There was no significant difference between the GC peaks in the PetroBrick extract and in the uninduced FabH2/PetroBrick Extract. All of the peaks in these two extracts that fall within the expected elution time of the C16 alkane( 9.5-10 min) correspond to trace amounts of compounds that we cannot identify. When FabH2 is induced, we see a strong new peak at approximetly 9.75 minutes. The MS spectra of this peak is highly consistent with C16 alkane. The overall ion fragmentation fingerprint is identical to that alkane. Identification as a C16 alkane is confirmed by the presence of a strong parent ion at a mass of 226, exactly the mass of the C16 alkane. This confirms production of C16 alkane. In addition, some C14 alkane production was observed. This is the first time that even chain length alkanes have been produced recombinately, and expands the PetroBrick system to be able to produce all of the alkanes in the range C13-C17. Note that this peak corresponds to only trace levels of alkane( approximately 1mg/L), and future optimization is needed to increase even chain length production levels.
References
1.Beta-ketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. Choi KH, Heath RJ, Rock CO. J Bacteriol. 2000 Jan;182(2):365-70.
2. http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA317177&Location=U2&doc=GetTRDoc.pdf
3. Branched-chain fatty acid biosynthesis in Escherichia coli. Smirnova N, Reynolds KA. J Ind Microbiol Biotechnol. 2001 Oct;27(4):246-51.
Parts Submitted
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K590034 FabH2] This part contains the FabH2 coding sequence, codon optimized for E. coli
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K590030 FabH2-ADC-AAR] This part expresses FabH2, as well as AAR and ADC, the two genes responsible for alkane production.
 "
"