Team:Washington/Alkanes/Future/Localization
From 2011.igem.org
(→Direct Fusion) |
(→Localization) |
||
| Line 5: | Line 5: | ||
=Localization= | =Localization= | ||
| - | To improve the efficiency of acyl-ACP conversion through the two-step pathway to alkane production, we are performing optimization through localization of the two catalytic proteins. Decrease of the distance the intermediates must travel between [http://partsregistry.org/Part:BBa_K590032 Acyl-ACP Reductase] (AAR) and [http://partsregistry.org/ | + | To improve the efficiency of acyl-ACP conversion through the two-step pathway to alkane production, we are performing optimization through localization of the two catalytic proteins. Decrease of the distance the intermediates must travel between [http://partsregistry.org/Part:BBa_K590032 Acyl-ACP Reductase] (AAR) and [http://partsregistry.org/Part:BBa_K590031 Aldehyde Decarbonylase] (ADC) is expected to improve overall alkane synthesis. |
Initially, we attempted a direct fusion of the two enzymes with a linker. Our main approach is to optimize alkane production by re-use of Biobrick parts from a previous iGEM team. For this, we are re-adapting Biobrick parts from 2010 [https://2010.igem.org/Team:Slovenia/PROJECT/biosynthesis/violacein Slovenia's zinc finger] violacein biosysnthesis project. By colocalization of the individual violacein producing enzymes in it's five-step pathway, they were able to increase their pigment production by ~6-fold. Our goal for alkane production optimization is to use the zinc finger localization method to significantly increase yields. | Initially, we attempted a direct fusion of the two enzymes with a linker. Our main approach is to optimize alkane production by re-use of Biobrick parts from a previous iGEM team. For this, we are re-adapting Biobrick parts from 2010 [https://2010.igem.org/Team:Slovenia/PROJECT/biosynthesis/violacein Slovenia's zinc finger] violacein biosysnthesis project. By colocalization of the individual violacein producing enzymes in it's five-step pathway, they were able to increase their pigment production by ~6-fold. Our goal for alkane production optimization is to use the zinc finger localization method to significantly increase yields. | ||
Revision as of 16:43, 28 September 2011
Localization
To improve the efficiency of acyl-ACP conversion through the two-step pathway to alkane production, we are performing optimization through localization of the two catalytic proteins. Decrease of the distance the intermediates must travel between [http://partsregistry.org/Part:BBa_K590032 Acyl-ACP Reductase] (AAR) and [http://partsregistry.org/Part:BBa_K590031 Aldehyde Decarbonylase] (ADC) is expected to improve overall alkane synthesis.
Initially, we attempted a direct fusion of the two enzymes with a linker. Our main approach is to optimize alkane production by re-use of Biobrick parts from a previous iGEM team. For this, we are re-adapting Biobrick parts from 2010 Slovenia's zinc finger violacein biosysnthesis project. By colocalization of the individual violacein producing enzymes in it's five-step pathway, they were able to increase their pigment production by ~6-fold. Our goal for alkane production optimization is to use the zinc finger localization method to significantly increase yields.
Direct Fusion
Our first approach was direct fusion of two proteins using a glycine-serine linker. We chose to construct both of the linear configurations of AAR to ADC linkage to test the effects of fusion by N and C terminus regions on either enzyme's catalytic performance. This resulted in both the AAR-to-ADC and ADC-to-AAR fusion products.
Zinc Finger Fusion
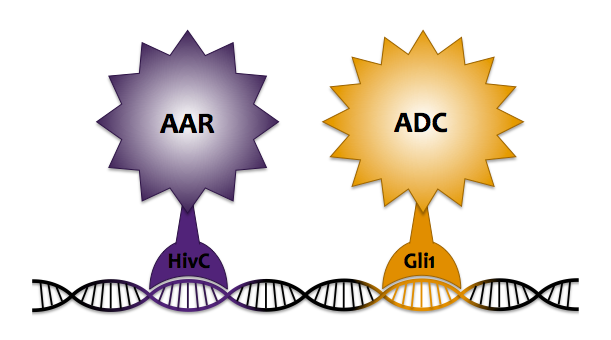
In an attempt to enhance our localization technique beyond the simple protein fusion method, we chose to integrate the use of DNA localization via zinc finger proteins to colocalize ADC and AAR to increase system flux. Zinc fingers are small DNA binding domains, consisting of around 30 amino acid residues folded around a single zinc cation. They function as transcriptional factors within eukaryotes, regulating endogenous gene expression by binding to specific sequences along DNA. Last year's Slovenia iGEM team characterized 6 zinc fingers and created a DNA program containing specific binding sites for each zinc finger. Our goal was to fuse AAR and ADC to their own respective zinc finger, such that when co-expressed with the DNA program, the fusion proteins would each bind to their specific target sequence along the DNA, thus being held in close proximity so that alkane production could be carried out more efficiently.
Results
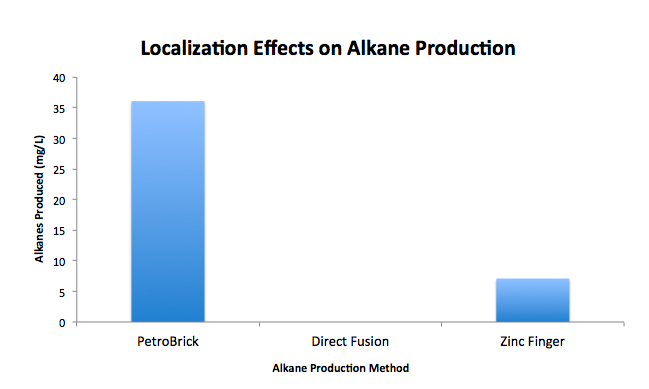
Contrary to what was expected, our GCMS data demonstrated that localization has a negative effect on alkane production. Our attempt at zinc finger localization resulted in an alkane production of approximately 7 mg/L, which is significantly less than our delocalized PetroBrick standard, which produced around 36 mg alkanes per liter. Because our alkane yields were low, we refrained from comprehensive testing of our localization constructs, so future groups are encouraged to refine our methods, as localization holds promise to theoretically increase the efficiency of this system.
 "
"