Team:UCL London/Manufacturing/PurityYieldCost/Analysis
From 2011.igem.org
| Line 10: | Line 10: | ||
[[File:Ucl-content-Manufacturing-figure3.jpg|center]] | [[File:Ucl-content-Manufacturing-figure3.jpg|center]] | ||
| + | |||
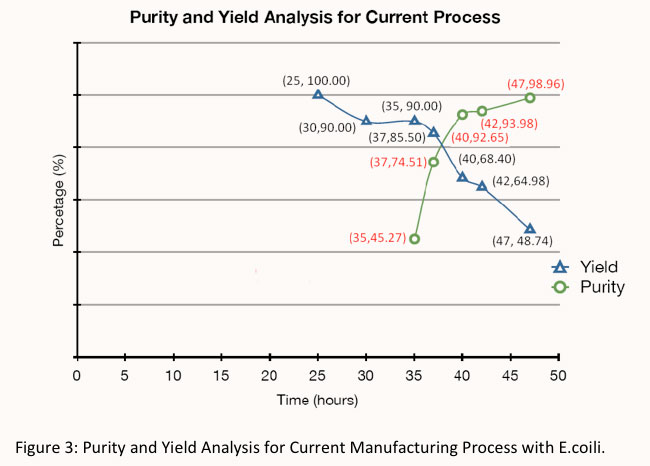
| + | For the current most commonly applied industrial bioprocess, the overall yield would be:<br /> | ||
| + | Yield of the process = 90% ×100% × 95% × 80% × 95% × 75% = '''48.74 %'''. | ||
| + | |||
| + | The yield, purity and processing time can be significantly improved if the E.coili cell factories with the five collaborating modules are applied. <br /> | ||
| + | Firstly, product titre will be higher compare to the fermentation of host strains without over-expression of gyrase. Gyrase is able to increase both quantity and quality of supercoiling, more functional plasmid molecules can be produced by bacteria cells. This leads to the consequence that less bioreactors are needed to produce the same amount of product, which reduces the initial capital investment massively.<br /> | ||
| + | In addition, the yield at UF/DF, chromatography steps can be raised due to the increased shear resistance of plasmid molecules. Also, since the product of interest is more homogeneous in terms of size and degree of supercoiling, its binding capacity will be more consistent for chromatography and the separation with impurities will be enhanced.<br /> | ||
| + | Last but not least, autolysis helps to ease the downstream processing, thus the purity of the product can be improved.<br /> | ||
| + | Thus the overall yield for the novel process with E.coili would be:<br /> | ||
| + | Yield of the process = 90% ×100% × 98% × 85% × 98% × 80% = '''58.78 %'''<br /> | ||
| + | A significant improvement of approximately 10% in yield could be achieved. | ||
| + | |||
| + | According to the FDA guidelines, the quality of plasmid DNA for clinical trials needs to achieve the approval specifications with undetectable proteins, RNAs and genomic DNAs [2]. The bioprocess with E.coili cell factory is able to achieve approximately 100% purity with two chromatography steps. However the current bioprocess can only reach 98.96% purity with the same down streaming processing, which indicates that more purification steps are needed to achieve satisfactory product purity. | ||
</div> | </div> | ||
{{:Team:UCL_London/Template/Footer}} | {{:Team:UCL_London/Template/Footer}} | ||
Revision as of 22:18, 21 September 2011
Analysing Results
Assume the yields of each steps are as labelled in the flow diagram [6], yield, purity and cost analyses were generated with the aid of SuperPro Designer® by Intelligen Inc. for both the current process and the process involved E.coili cell factory.
For the current most commonly applied industrial bioprocess, the overall yield would be:
Yield of the process = 90% ×100% × 95% × 80% × 95% × 75% = 48.74 %.
The yield, purity and processing time can be significantly improved if the E.coili cell factories with the five collaborating modules are applied.
Firstly, product titre will be higher compare to the fermentation of host strains without over-expression of gyrase. Gyrase is able to increase both quantity and quality of supercoiling, more functional plasmid molecules can be produced by bacteria cells. This leads to the consequence that less bioreactors are needed to produce the same amount of product, which reduces the initial capital investment massively.
In addition, the yield at UF/DF, chromatography steps can be raised due to the increased shear resistance of plasmid molecules. Also, since the product of interest is more homogeneous in terms of size and degree of supercoiling, its binding capacity will be more consistent for chromatography and the separation with impurities will be enhanced.
Last but not least, autolysis helps to ease the downstream processing, thus the purity of the product can be improved.
Thus the overall yield for the novel process with E.coili would be:
Yield of the process = 90% ×100% × 98% × 85% × 98% × 80% = 58.78 %
A significant improvement of approximately 10% in yield could be achieved.
According to the FDA guidelines, the quality of plasmid DNA for clinical trials needs to achieve the approval specifications with undetectable proteins, RNAs and genomic DNAs [2]. The bioprocess with E.coili cell factory is able to achieve approximately 100% purity with two chromatography steps. However the current bioprocess can only reach 98.96% purity with the same down streaming processing, which indicates that more purification steps are needed to achieve satisfactory product purity.
 "
"