Team:EPF-Lausanne/Our Project/Data
From 2011.igem.org
(→Readout system - TetR, LacI and RFP) |
|||
| Line 95: | Line 95: | ||
| - | The plasmids used here are pSB3K1 pConst-TetR and J61002 Ptet-RFP. For more details about them, please refer to the | + | The plasmids used here are pSB3K1 pConst-TetR and J61002 Ptet-RFP. For more details about them, please refer to the [https://2011.igem.org/Team:EPF-Lausanne/Our_Project/Assembly Reporter plasmids] page. |
| Line 120: | Line 120: | ||
[[File:EPFL_Summary_TetR_LacI_RFP.jpg|700px]] | [[File:EPFL_Summary_TetR_LacI_RFP.jpg|700px]] | ||
| - | To get this readout system, we cotransformed pSB3K1 Pconst-TetR Ptet-LacI with J61002 Plac-RFP. Unfortunately, the sequence of Ptet in front of LacI got mutated during the assembly process, resulting in only a partial repression of LacI by TetR. Still, our results do show the effects of TetR and LacI on the whole system. For more details about the plasmids, please refer to the | + | To get this readout system, we cotransformed pSB3K1 Pconst-TetR Ptet-LacI with J61002 Plac-RFP. Unfortunately, the sequence of Ptet in front of LacI got mutated during the assembly process, resulting in only a partial repression of LacI by TetR. Still, our results do show the effects of TetR and LacI on the whole system. For more details about the plasmids, please refer to the [https://2011.igem.org/Team:EPF-Lausanne/Our_Project/Assembly Reporter plasmids] page. |
Revision as of 17:40, 18 September 2011
Data
team, please check this page: [1]
Contents |
New parts
we must have 3 favourite, then the others come in a "other biobrick" section
Medium-strength Plac
blablabla
TetR Mutants
- V36F
Sequence
ATGTCCAGATTAGATAAAAGTAAAGTGATTAACAGCGCATTAGAGCTGCTTAATGAGGTCGGAATCGAAGGTTTAACAACCCGTAAACTCGCCCAGAAGCTAGGTTTCGAGCAGCCTACATTGTATTGGCATGTAAAAAATAAGCGGGCTTTGCTCGACGCCTTAGCCATTGAGATGTTAGATAGGCACCATACTCACTTTTGCCCTTTAGAAGGGGAAAGCTGGCAAGATTTTTTACGTAATAACGCTAAAAGTTTTAGATGTGCTTTACTAAGTCATCGCGATGGAGCAAAAGTACATTTAGGTACACGGCCTACAGAAAAACAGTATGAAACTCTCGAAAATCAATTAGCCTTTTTATGCCAACAAGGTTTTTCACTAGAGAATGCATTATATGCACTCAGCGCTGTGGGGCATTTTACTTTAGGTTGCGTATTGGAAGATCAAGAGCATCAAGTCGCTAAAGAAGAAAGGGAAACACCTACTACTGATAGTATGCCGCCATTATTACGACAAGCTATCGAATTATTTGATCACCAAGGTGCAGAGCCAGCCTTCTTATTCGGCCTTGAATTGATCATATGCGGATTAGAAAAACAACTTAAATGTGAAAGTGGGTCT
- P39K
- Y42F
- P39Q-Y42M
Sequence
ATGTCCAGATTAGATAAAAGTAAAGTGATTAACAGCGCATTAGAGCTGCTTAATGAGGTCGGAATCGAAGGTTTAACAACCCGTAAACTCGCCCAGAAGCTAGGTGTAGAGCAGCAAACAGTGATGTGGCATGTAAAAAATAAGCGGGCTTTGCTCGACGCCTTAGCCATTGAGATGTTAGATAGGCACCATACTCACTTTTGCCCTTTAGAAGGGGAAAGCTGGCAAGATTTTTTACGTAATAACGCTAAAAGTTTTAGATGTGCTTTACTAAGTCATCGCGATGGAGCAAAAGTACATTTAGGTACACGGCCTACAGAAAAACAGTATGAAACTCTCGAAAATCAATTAGCCTTTTTATGCCAACAAGGTTTTTCACTAGAGAATGCATTATATGCACTCAGCGCTGTGGGGCATTTTACTTTAGGTTGCGTATTGGAAGATCAAGAGCATCAAGTCGCTAAAGAAGAAAGGGAAACACCTACTACTGATAGTATGCCGCCATTATTACGACAAGCTATCGAATTATTTGATCACCAAGGTGCAGAGCCAGCCTTCTTATTCGGCCTTGAATTGATCATATGCGGATTAGAAAAACAACTTAAATGTGAAAGTGGGTCT
T7 promoter variants
Pre-existing parts
add something about the lysis cassette, and add also on its page on the partsregistry!
DNA recovery
Vincent, you could drop your nice explanations & graphs in here :) I guess the "Characterization of Variants Using Fluorescence" should go with the T7 promoters biobricks
T7 Promoter Variants
Characterization of Variants Using Fluorescence
For each family, we tested the randomers and the designed variants separately. To characterize the promoter strengths, we used RFP as the reporter gene and used a platereader to test for fluorescence during and after induction with IPTG.
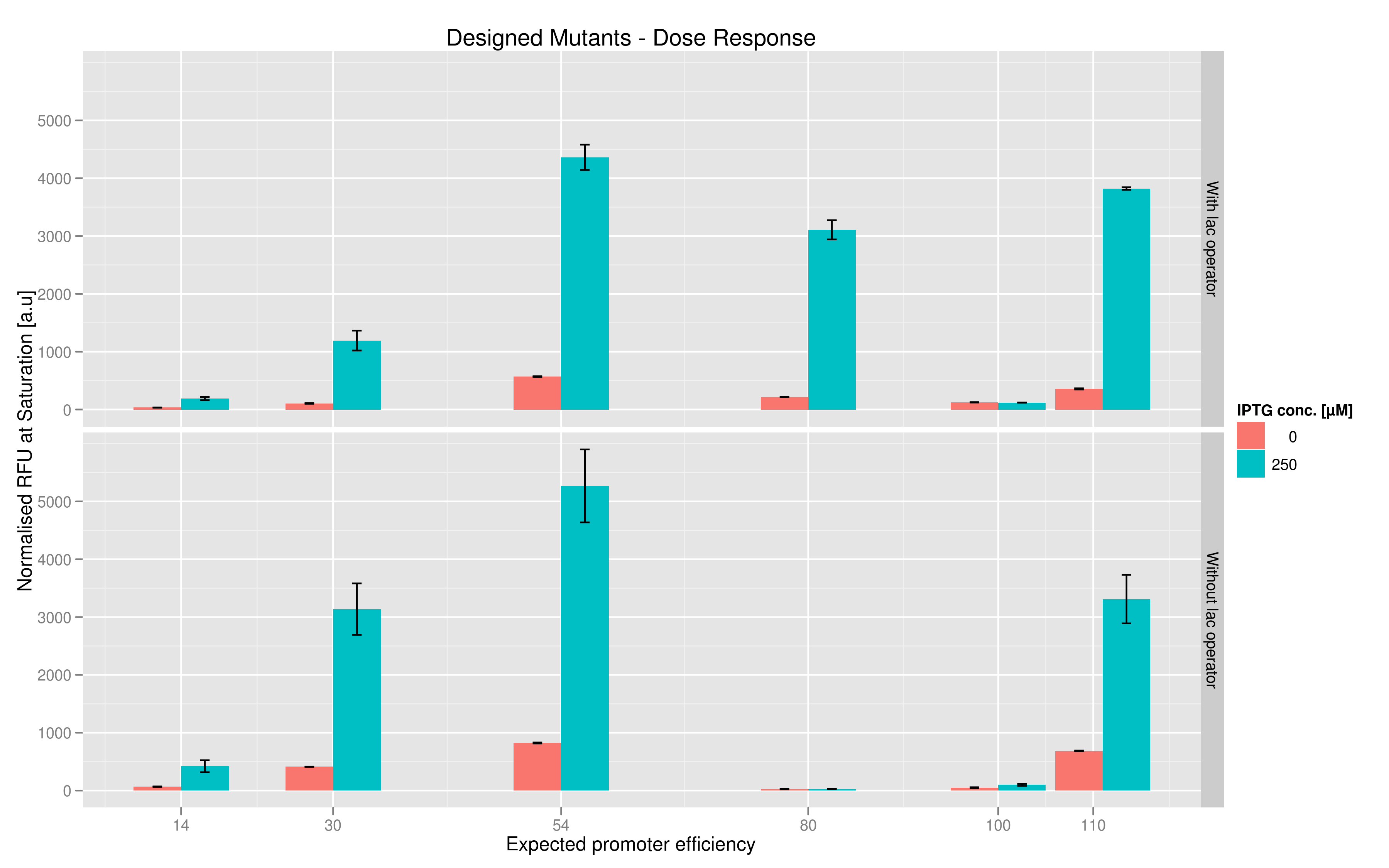
The six designed T7 promoter variants are named as a function of their predicted promoter efficiency, relative to the wildtype. For example, T7 14 has a predicted efficiency of 14% compared to the consensus T7 promoter, whereas T7 111 is predicted to be 111% more efficient than the wildtype. In the chart above, you find each of the designed promoter variants for both the T7 with and without the lac operator, arranged in increasing predicted efficiency. Contrary to our expectation, some variants that were designed to have a lesser efficiency than the wildtype (e.g. T7 54) seem to have a much higher strength (as measured by fluorescence at saturation, normalized by the optical density). Already in the designed variants, we see a substantial difference in the behavior of the promoters that have a lac operator as opposed to those that do not. The data for this graph was produced in triplicate, so the error bar represents the standard error across those three measurements.
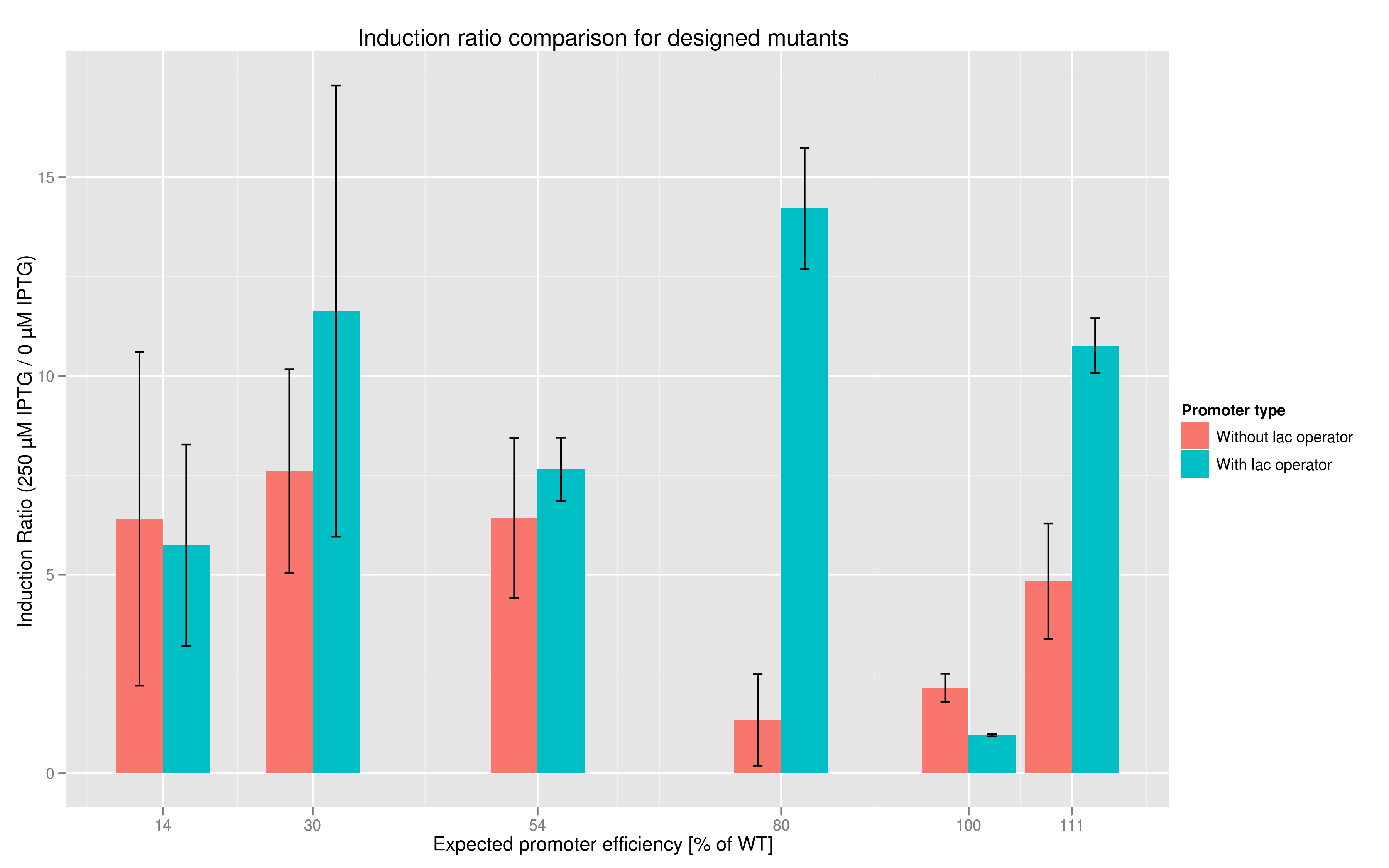
In addition to fluorescence at saturation, another way to characterize promoter strength is to look at its induction ratio, which is the ratio, at saturation, of fluorescence produced by induction with IPTG versus fluorescence produced without induction. In layman's terms, it indicates how strongly the promoter reacts to induction. Here again, our results indicate that some promoter variants (T7 80 in particular) stand out with respect to this feature. Here too the importance of the lac operator in producing high induction ratios is not to be underestimated.
For the three sets of randomers for T7 with and without the lac operator, we tested seventy-two different variants and characterized their expression using the same IPTG induction protocol as with the designed variants. The goal of using these variants was to examine the range of expressions that can be produced by random mutations as opposed to directed mutations. The results, as presented in the graph, indicate that the designed variants (with and without lac operator put together) produce a much higher average normalized fluorescence than the randomers.
Characterization and DNA Recovery with Lysis
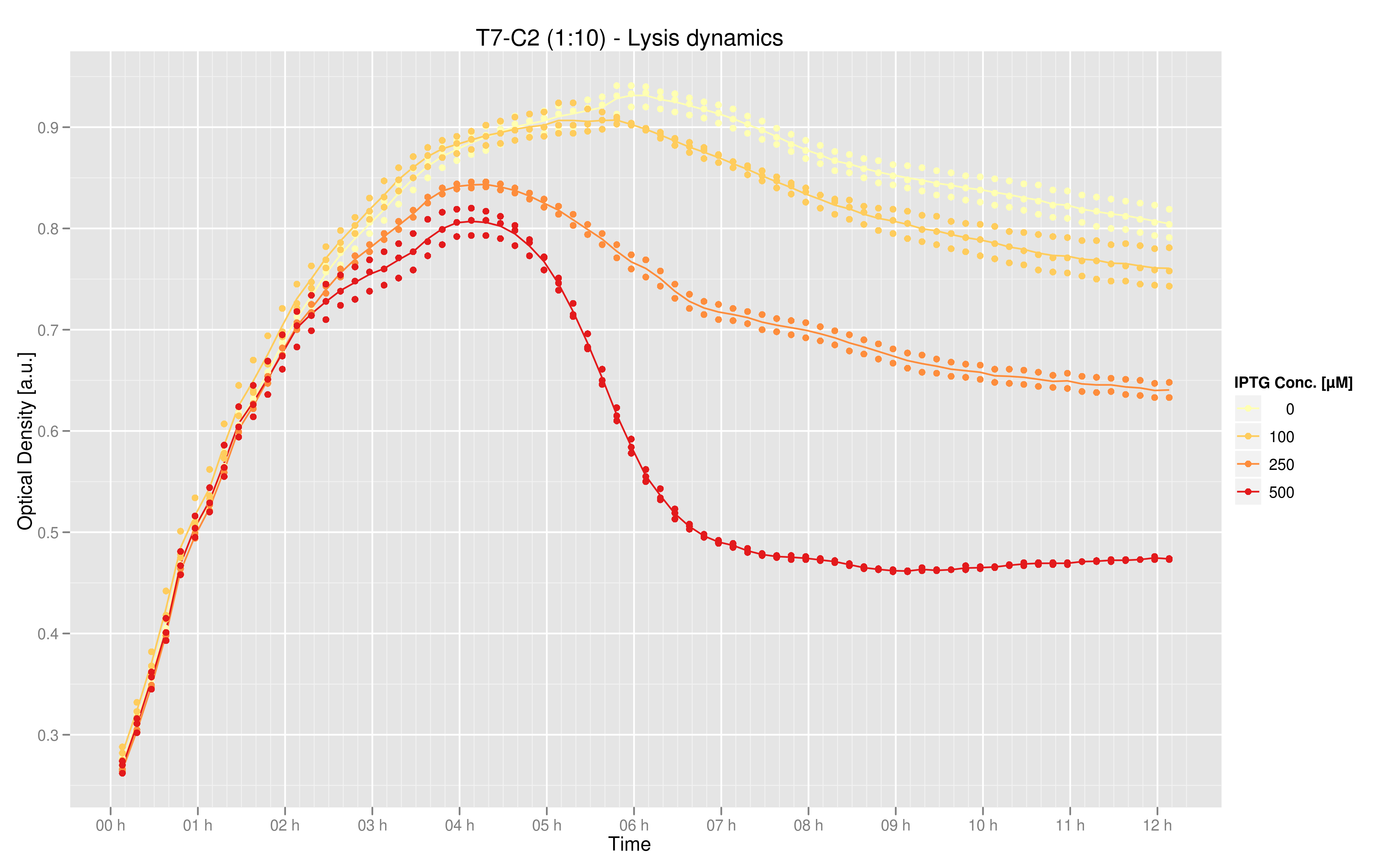
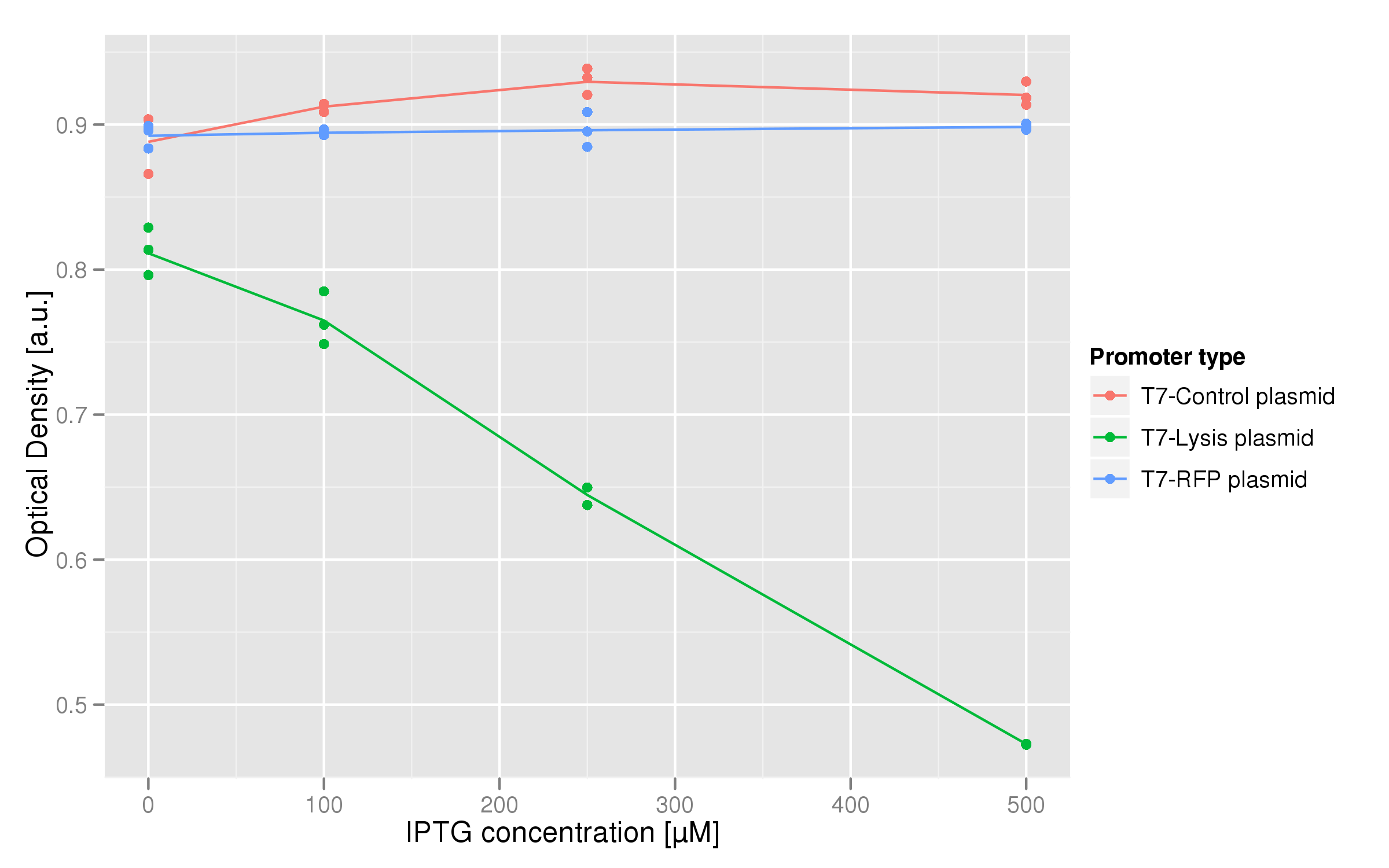
Since a major component of our scheme for selecting promoters and transcription factors with strong binding affinities required the ability to lyse cells, we also wanted to test a T7-driven lysis cassette.
Induction with various concentrations of IPTG reveals a stready increase in the amount of lysis that can be obtained. Here 500 uM yields the most substantial amount of lysis, and that concentration was used in all further experiments dealing with lysis. The negative controls were two-fold: one is an unsuccessful attempt at inserting the lysis cassette downstream of a T7 promoter in the psB3K1 plasmid, while the other is a T7 promoter upstream of an RFP gene. Neither should express lysis.
In vivo characterization - Readout systems
J61002 Ptet-RFP
We are showing the data of RFP induction in cells containing J61002 Ptet-RFP, as a reference to compare the data of the first readout system (TetR and RFP), where the cells contain both J61002 Ptet-RFP and pSB3K1 Pconst-TetR.
ATC induction
[[File:]]
Dose-response
[[File:]]
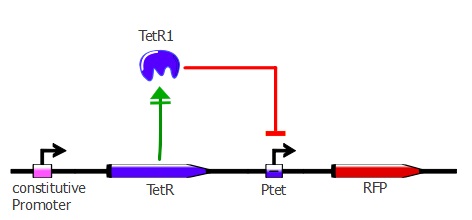
Readout system - TetR and RFP
The first readout system is composed of TetR with a constitutive promoter, followed by RFP with a Ptet promoter. If TetR binds to Ptet, then RFP is repressed. This readout system is convenient for fluorescence detection experiments, but it would not be suited for using the lysis cassette as the reporter gene is being repressed upon TetR-Ptet interaction. With the lysis device, the interesting cells (where TetR binds to Ptet) would survive and we would recover only the useless TetR mutants.
The plasmids used here are pSB3K1 pConst-TetR and J61002 Ptet-RFP. For more details about them, please refer to the Reporter plasmids page.
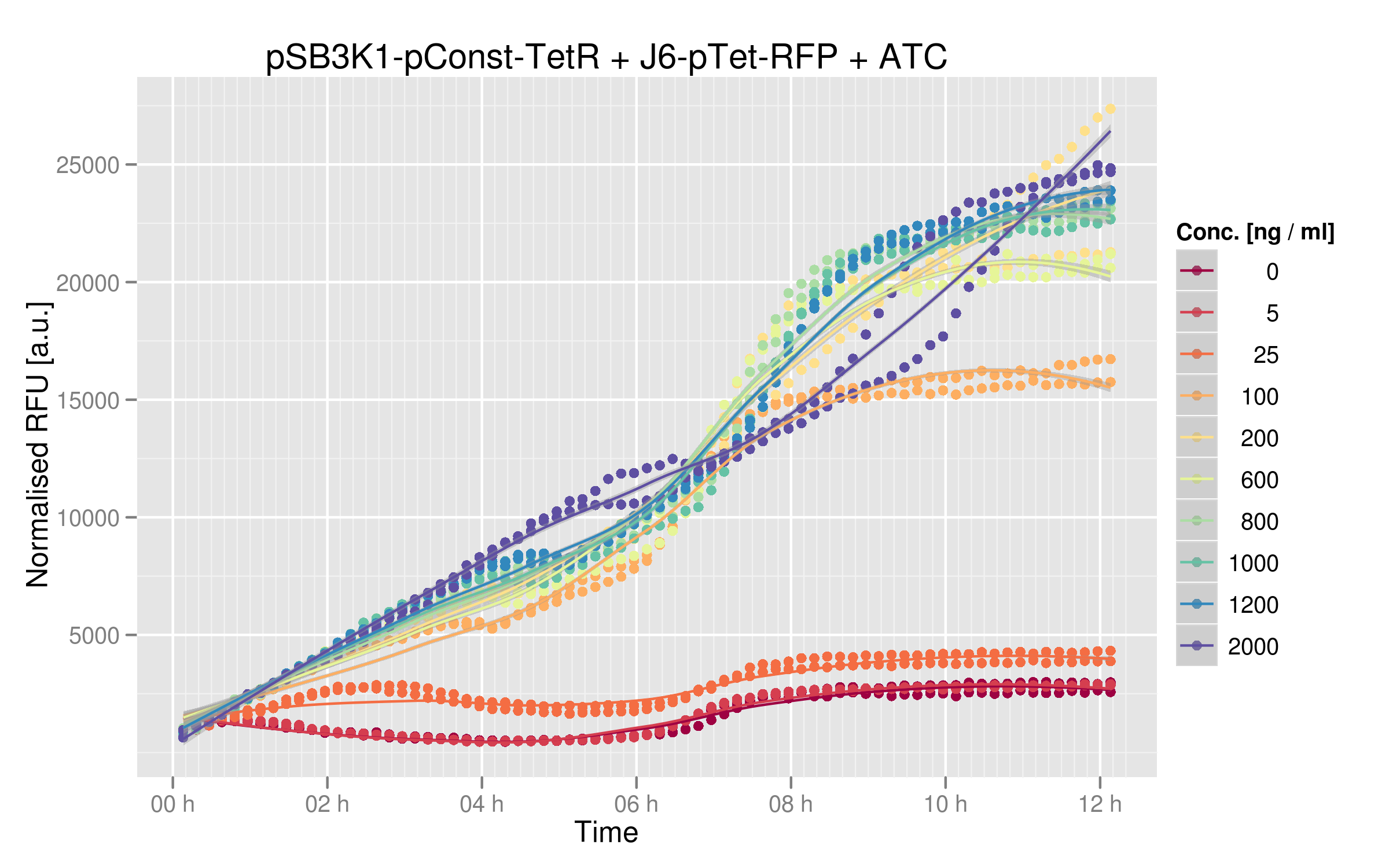
ATC induction
When adding ATC to the co-transformed cells, TetR is repressed and cannot stop RFP anymore, so RFP increases.
The induction curves show that RFP indeed increases with addition of TetR. Without any ATC in the medium, cells express about 2500 normalized RFUs when they reach a plateau, whereas with the highest values of ATC we have 25'000 normalized RFUs. There is a 10x difference between normal medium and additon of ATC, showing that our first readout system is effectively powerful: TetR mutants that would not recognize the consensus Ptet sequence would yield a lot more RFP expression than other mutants recognizing Ptet.
Dose-response
By looking at the dose-response graph, we can see a significant increase between 0 and 200 ng/microL ATC; then the RFUs are quite stable. Here again, the interaction TetR-Ptet has a strong impact on the output (i.e. normalized RFUs).
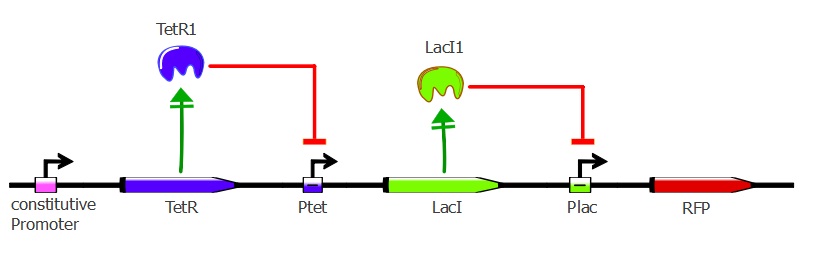
Readout system - TetR, LacI and RFP
This second readout system is composed of 3 genes: TetR under Pconst control, LacI under Ptet control (playing the role of an inverter) and finally RFP under pLac control. Here, RFP is induced when TetR binds to pTet. Lysis cassette can be put instead of RFP in thy system, having as a consequence that the cells where TetR mutants bind to pTet would lyse.
To get this readout system, we cotransformed pSB3K1 Pconst-TetR Ptet-LacI with J61002 Plac-RFP. Unfortunately, the sequence of Ptet in front of LacI got mutated during the assembly process, resulting in only a partial repression of LacI by TetR. Still, our results do show the effects of TetR and LacI on the whole system. For more details about the plasmids, please refer to the Reporter plasmids page.
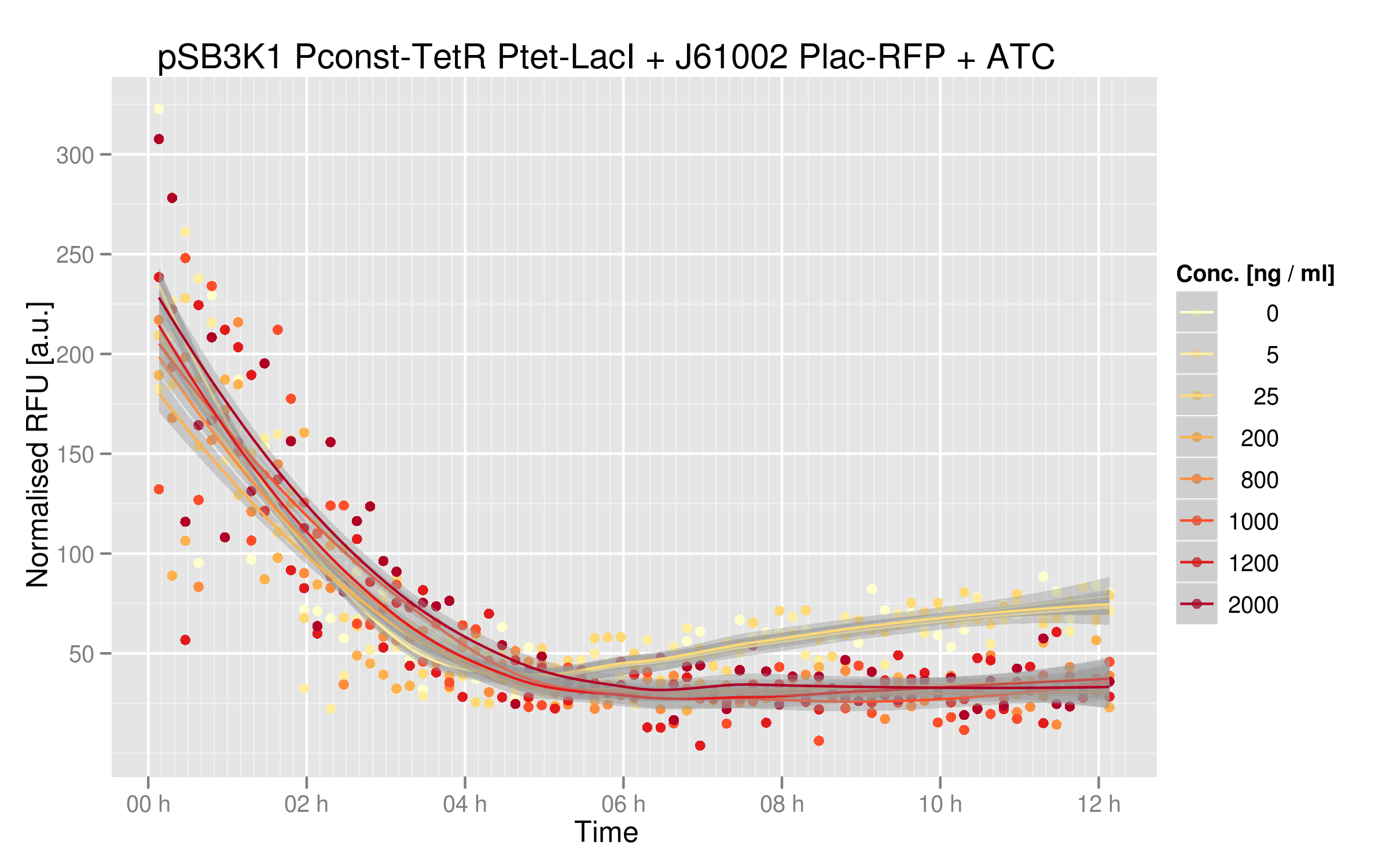
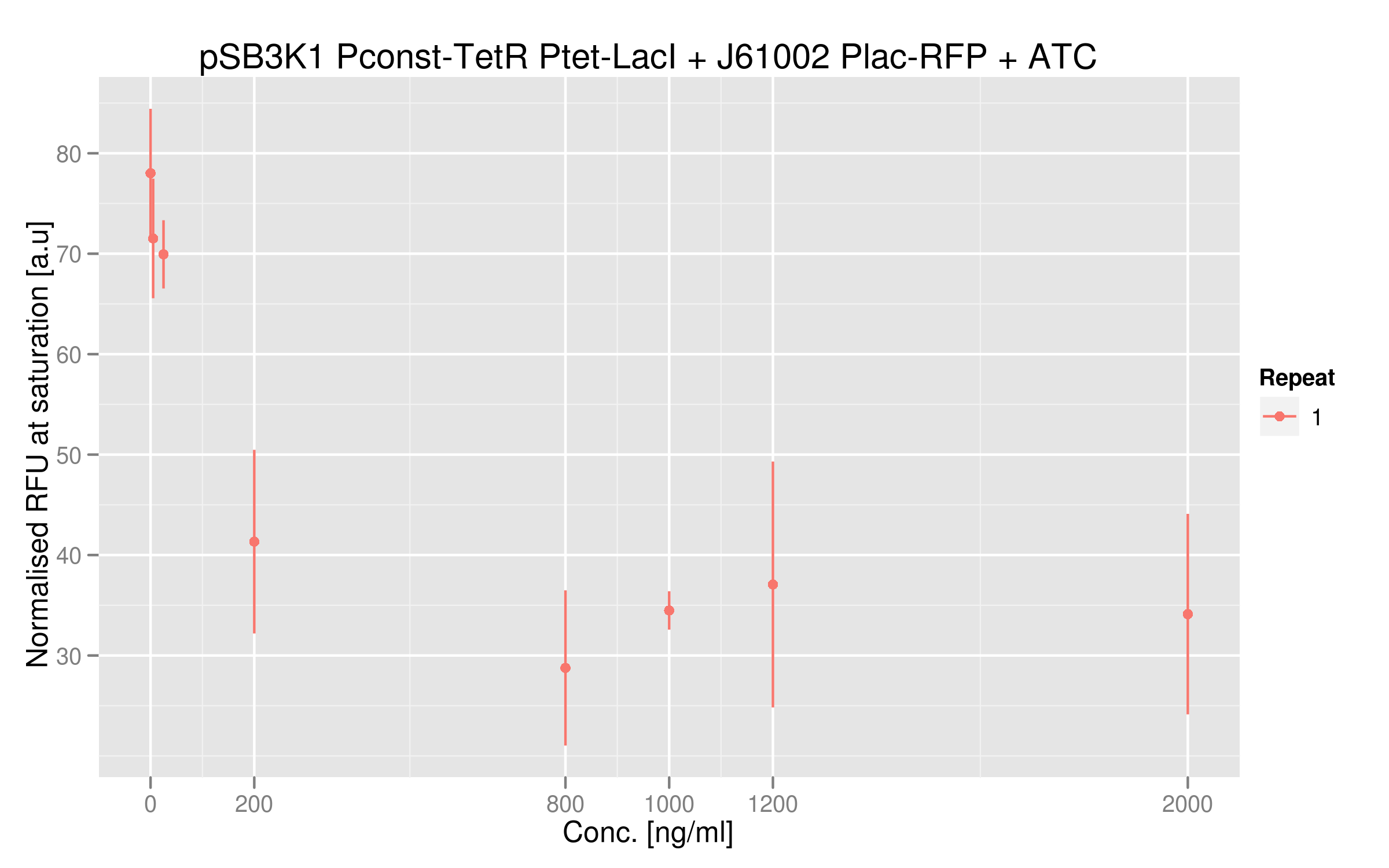
ATC induction
Adding ATC in the medium of the cotransformed cells will inactivate TetR. As a consequence, LacI will be expressed and RFP will be repressed. We should then see a decrease of RFUs correlating with increasing concentrations of ATC.
With no or low concentrations of IPTG in the cells' medium, RFP expression is quite weak; this can be explained by the fact that Ptet was mutated in our plasmids and thus TetR couldn't repress LacI very efficiently. In normal conditions, we should see RFP expression when TetR binds to Ptet - which is the case here, since we have the wild-type TetR gene. Here, LacI not well repressed by TetR, thus RFP is repressed even when TetR binds to Ptet. Nevertheless, there is a decrease in RFP expression when we add sufficient amounts of ATC. Even in our mutated system, TetR interaction to Ptet still has an effect on the output. There is a 2-fold difference between high ATC concentrations and no ATC; we believe that, by restoring Ptet sequence, this difference would be higher.
ATC dose-response curve
This data shows more clearly the difference of normalized RFUs for low or high concentrations of ATC - even if the intensities are low. As in our first readout system, the highest ATC efficiency seems to be reached with 200 ng/microL already.
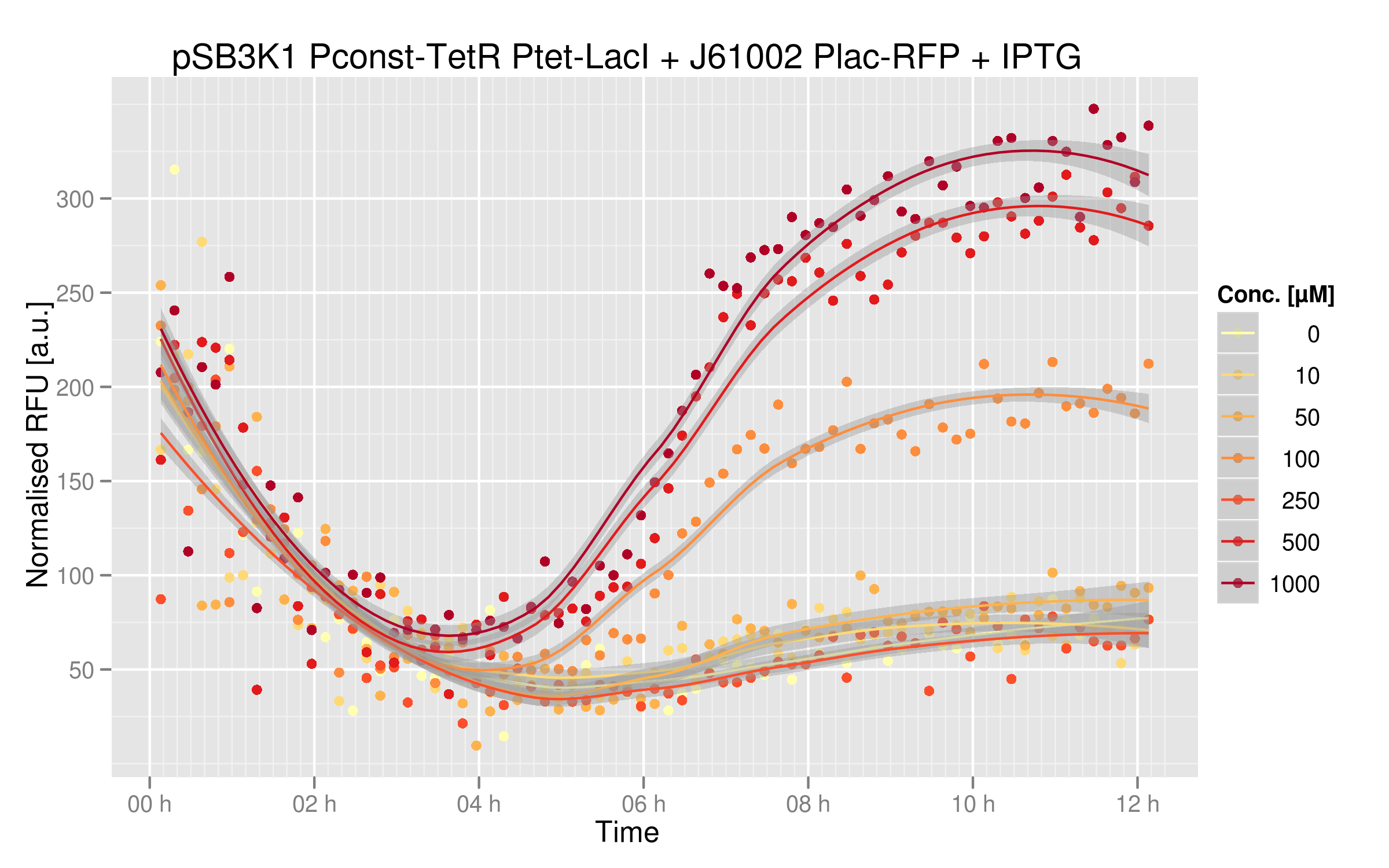
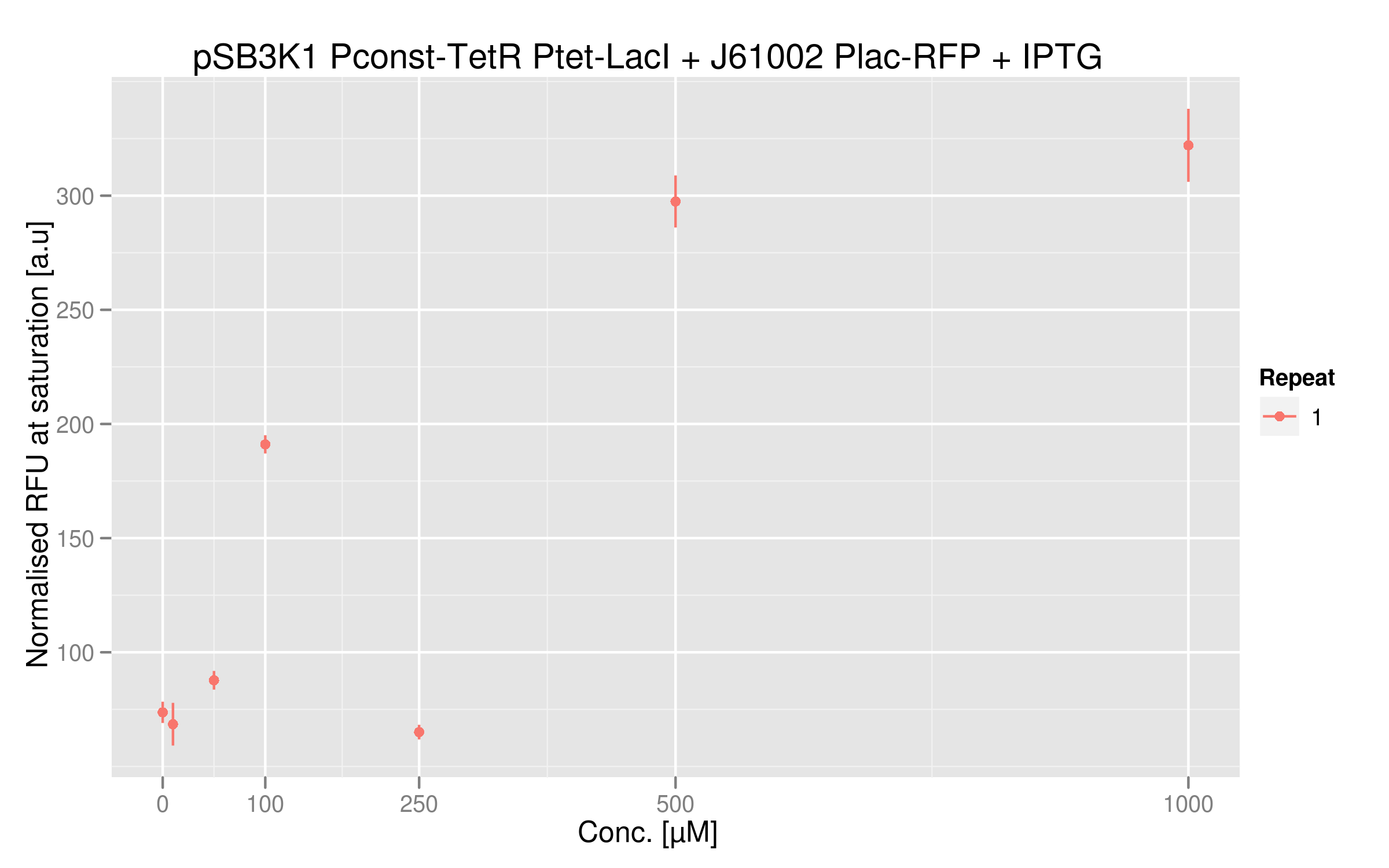
IPTG induction
IPTG will inactivate LacI, so that RFP will be more expressed. The expected results are an increase of RFUs in parallel of an increase of IPTG concentration in the medium.
Indeed,
IPTG dose-response curve
 "
"