Team:Paris Bettencourt/MultiHost
From 2011.igem.org
(→= Cloning in K606003) |
(→Cloning in K606003) |
||
| Line 42: | Line 42: | ||
K606003 is not a synthetic biology plasmid. It is not silent, and it is a bit more difficult to clone inside than a regular registry plasmid. We have troubledshooted this plasmid a lot and we can now gives good advices for a good method to clone in this vector. | K606003 is not a synthetic biology plasmid. It is not silent, and it is a bit more difficult to clone inside than a regular registry plasmid. We have troubledshooted this plasmid a lot and we can now gives good advices for a good method to clone in this vector. | ||
| - | The vector is ampicilyn resistant. The resistance casette is a bit stronger than a standard resistance casette. We advise you to put a bit more antibiotic than usual. Ampicilyn is an antibiotic that is destroyed outside the cells. If the bacteria are too concentrated, the ampicilyn disapears quickly, and the bacteria loose it's plasmid (that is already big). That's why, one have to care not to grow the bacteria for a too long time when minipreping, and to take well insulated colonies when picking on the plate. Beware, | + | The vector is ampicilyn resistant. The resistance casette is a bit stronger than a standard resistance casette. We advise you to put a bit more antibiotic than usual. Ampicilyn is an antibiotic that is destroyed outside the cells. If the bacteria are too concentrated, the ampicilyn disapears quickly, and the bacteria loose it's plasmid (that is already big). That's why, one have to care not to grow the bacteria for a too long time when minipreping, and to take well insulated colonies when picking on the plate. Beware, satellites colonies appears fast due to the strengh of the ampicilyn casette. They do not contain the plasmid. |
| + | |||
| + | |||
| + | <table> | ||
| + | <td> | ||
| + | <tr>[[File:pHM3_when_plated_too_dense.jpg]]</tr> | ||
| + | </td> | ||
| + | |||
Here is a protocol we advise you to follow. Once your ligation is done and plated, pick up 10 colonies, that you grow in LB and make PCR colony on in the mean time. Use the primersgiven above. From the result of the PCR, select the goods tubes that indeed contains your insert, replicate them for glycerols, miniprep them for gl and sequence. Your cloning will be sucessful each time! | Here is a protocol we advise you to follow. Once your ligation is done and plated, pick up 10 colonies, that you grow in LB and make PCR colony on in the mean time. Use the primersgiven above. From the result of the PCR, select the goods tubes that indeed contains your insert, replicate them for glycerols, miniprep them for gl and sequence. Your cloning will be sucessful each time! | ||
Revision as of 09:27, 3 September 2011

Contents |
Description
A new vector
There were one multi host vector in the registry, but they were not able to send it to us. The one we received finally was not able to growth in culture. So we had to build our new plasmid for our experiments.
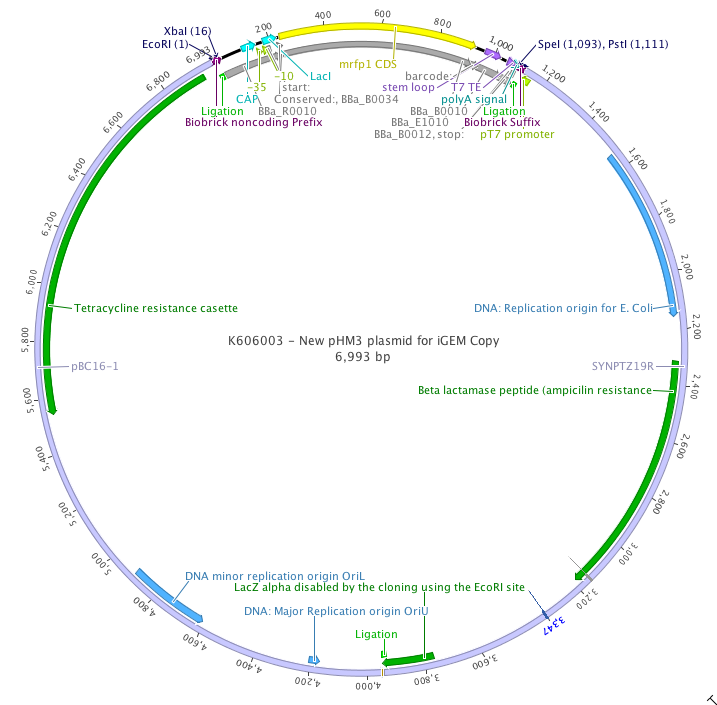
This plasmid was obtained thanks to Harald Putzer's laboratory at IBPC, and in the corresponding publication, hold the name of pHM3. This is the fusion of two commercial plasmids via their EcoRI site. The pBC16-1 plasmid is a commercial low copy plasmid for Bacillus Subtilis. SynPTZ19R is a medium copy plamid for E. Coli.
Biobricking
As there were two EcoRI site, we had to remove one of them. This was done by menaged digestion and completion using Taq poylmerase. The blunt ends were then religated. In order to make this plasmid biobricked and in order to have a negative digestion control in the frame of the new 2011 cloning method, we inserted the biobrick J0445 inside . By doing that we recovered the EcoRI, XbaI, SpeI and PstI site of the biobrick format. The plasmid map is now the following:
Sequencing
The vector was partially sequenced and it shows correct sequences on the parts sequenced.
We sequenced several time with sucess the biobrick inserted using these primers:
K606003-Fwd: GTATATAAACATTCTCAAAGG
K606003-Rev: GGATAACAATTTCACACAGG
Caracterization
Miniprep preparation
An overnight miniprep of this plasmid in E. Coli ofter gives yields around 200ng/µL which is lower than the standards plasmid of the database that gives around 500-800 ng/µL in the same conditions. This confirms the plasmid is medium copy in E. Coli.
Sequence analysis
The plasmid is found out to have a pT7 promoter in the reverse direction compared to the biobrick. It may cause a problem wih construction holding a T7 polymerase
Cloning in K606003
K606003 is not a synthetic biology plasmid. It is not silent, and it is a bit more difficult to clone inside than a regular registry plasmid. We have troubledshooted this plasmid a lot and we can now gives good advices for a good method to clone in this vector.
The vector is ampicilyn resistant. The resistance casette is a bit stronger than a standard resistance casette. We advise you to put a bit more antibiotic than usual. Ampicilyn is an antibiotic that is destroyed outside the cells. If the bacteria are too concentrated, the ampicilyn disapears quickly, and the bacteria loose it's plasmid (that is already big). That's why, one have to care not to grow the bacteria for a too long time when minipreping, and to take well insulated colonies when picking on the plate. Beware, satellites colonies appears fast due to the strengh of the ampicilyn casette. They do not contain the plasmid.
 "
"