Team:Paris Bettencourt/Designs
From 2011.igem.org
| Line 21: | Line 21: | ||
<p>The aim of this state is to characterize the nanotube communication. The idea is to pass molecules of different sizes and to measure the interval of time between the apparition of the two monitors which is linked to the diffusion time through the nanotube.</p> | <p>The aim of this state is to characterize the nanotube communication. The idea is to pass molecules of different sizes and to measure the interval of time between the apparition of the two monitors which is linked to the diffusion time through the nanotube.</p> | ||
| - | <a href="https://2011.igem.org/File:Step1_principle.jpg"><img src="../wiki/images/1/1c/Step1_principle.jpg" style="width: 400px; margin: 15px; margin-left: | + | <a href="https://2011.igem.org/File:Step1_principle.jpg"><img src="../wiki/images/1/1c/Step1_principle.jpg" style="width: 400px; margin: 15px; margin-left: 25%;"></a></br> |
[[File:Step1_principle.jpg|thumb|center|upright=3.0|General plan for the experiment design to characterize the nanotubes]] | [[File:Step1_principle.jpg|thumb|center|upright=3.0|General plan for the experiment design to characterize the nanotubes]] | ||
Revision as of 22:17, 25 August 2011

Here is the design page, in which are summed up all the potential designs we may realize.
Designs for a direct observation
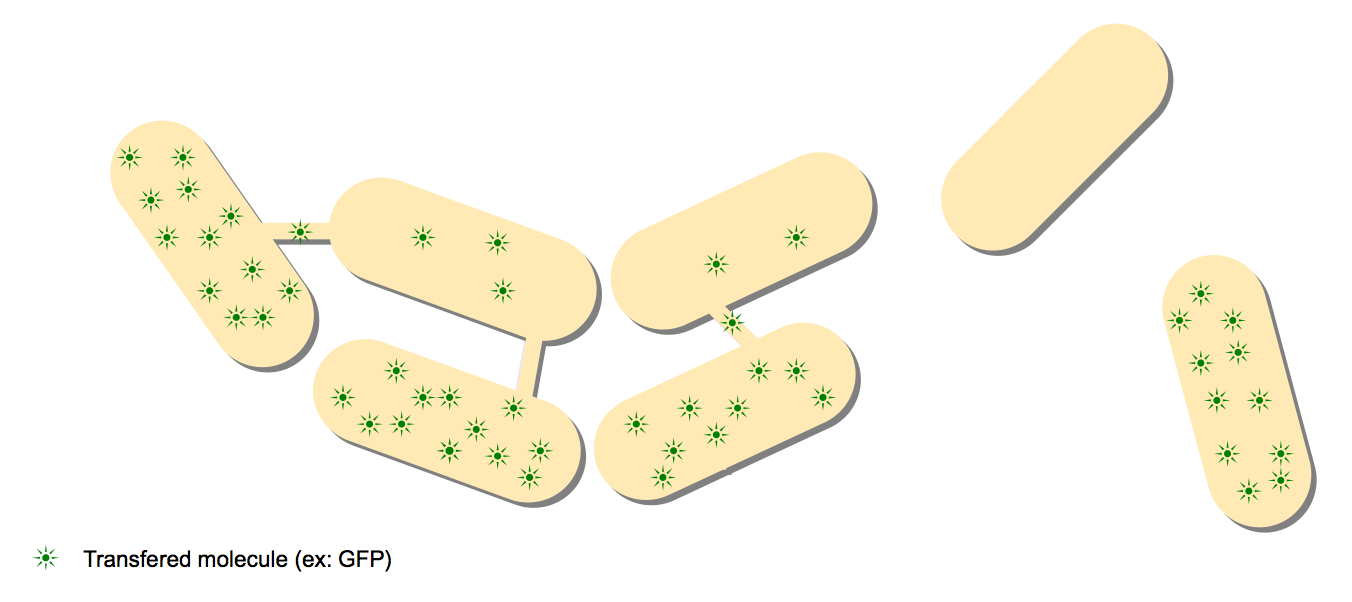
The principle of these designs is to observe directly the molecule that passes from a producer cell to a receptor cell through the nanotubes. There is no signal amplification in this step.
We currently have 2 designs for it:
- [[Team:Paris_Bettencourt/Antibiotic_diffusion|The Antibiotics resistance experiences:]] The principle is to put two strains of bacteria that present different antibiotic resistances in the same biofilm. After a growth time together, they are exposed to the two antibiotics corresponding to the resistances. They can resist together through a cooperative effect involving the exchange of antibiotic resistance enzymes through the nanotubes.
- [[Team:Paris_Bettencourt/GFPLac_diffusion|The GFP-LacI fusion:]] The principle is to diffuse a GFP-LacI fusion protein from one cell that produces it to a neighboring cell that is without color and contains a plasmid with numerous LacO operons. The GFP that enters the neighboring cell via the nanotubes will then be concentrate on the plasmid giving a more intense fluorescence.
Designs for nanotube characterization
The aim of this state is to characterize the nanotube communication. The idea is to pass molecules of different sizes and to measure the interval of time between the apparition of the two monitors which is linked to the diffusion time through the nanotube.
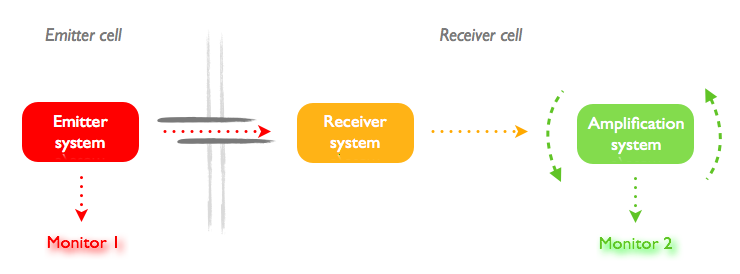 [[File:Step1_principle.jpg|thumb|center|upright=3.0|General plan for the experiment design to characterize the nanotubes]]
[[File:Step1_principle.jpg|thumb|center|upright=3.0|General plan for the experiment design to characterize the nanotubes]]
First, we put the two constructions in the same cell and induce their expression with different quantities of IPTG. Then we measure the time between the apparition of monitor 1 (RFP) and monitor 2 (GFP). We repeat the experiment, but with the two constructs separately introduced into two different cells. There should be an increase in the delay of apparition of the second monitor due to the necessary diffusion time through the nanotube.
We expect the time to increase with the size of the molecule as the diffusion coefficient is D = K/R where K is a constant and R the Stoke radius.
We try to demonstrate and characterize the communication through the nanotube system for Bacillus Subtilis, but also between B. Subtilis and E. Coli. The designs are classified by type of demonstration.
Design for Subtilis-Subtilis communication
In B. Subtilis, the nanotubes directly connect the cytoplasms of the two cells. Thus, simple designs with cytoplasmic proteins can be used.
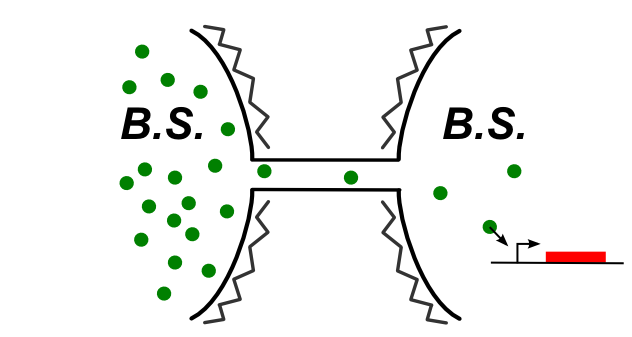 [[File:cytoplasm_cytoplasm_communication.jpg|thumb|center|upright=2.0|Schematic of the supposed connection between the cells via the nanotubes]]
[[File:cytoplasm_cytoplasm_communication.jpg|thumb|center|upright=2.0|Schematic of the supposed connection between the cells via the nanotubes]]
The designs we have made so far are the following, classified by the size of the molecule we want to pass through the tubes:
- [[Team:Paris_Bettencourt/T7_diffusion|The T7 diffusion:]] The principle of this experiment is to pass T7 polymerase through the nanotubes, this T7 activating the T7 amplifier system in the receptor cell.
- [[Team:Paris_Bettencourt/Xis_diffusion|The Xis protein diffusion:]] Xis is a small partner of an exisase. The latter will exise a stop codon on the DNA strand that is preventing the expression of the GFP.
- [[Team:Paris_Bettencourt/ComS_diffusion|The ComS diffusion:]] The idea is to trigger the switch of the MeKS system by diffusing ComS through the nanotubes.
- [[Team:Paris_Bettencourt/tRNA_diffusion|The amber suppressor tRNA diffusion:]] The principle of this design is to produce in one cell a amber supressor tRNA that will diffuse through the nanotubes. The receptor cell holds the gene for a T7 with amber stop codons that cannot be translated into a functional protein as long as the tRNA amber suppressor is not present in the cell. Once expressed, the T7amber will trigger the T7 amplification system.
- [[Team:Paris_Bettencourt/Xylose_diffusion|The xylose diffusion:]] Xylose accumulated in one of the cell diffuse through the tubes and trigger the amplifier in the receiver cell.
Designs for B. Subtilis-E. Coli communication
We want to demonstrate that communication through nanotubes can be established between Gram+ and Gram- bacteria. This would be the first demonstration of protein exchange between species using this newly discovered means of communication. E. Coli will be used as a model for Gram- bacteria. However, we don't know if this communication exists, if it will occur with the periplasm or directly with the cytoplasm. We have two type of designs.
If communication happens with the cytoplasm
If the communication happens directly with the cytoplasm, we can basically use the same systems used for the Subtilis-Subtilis communication.
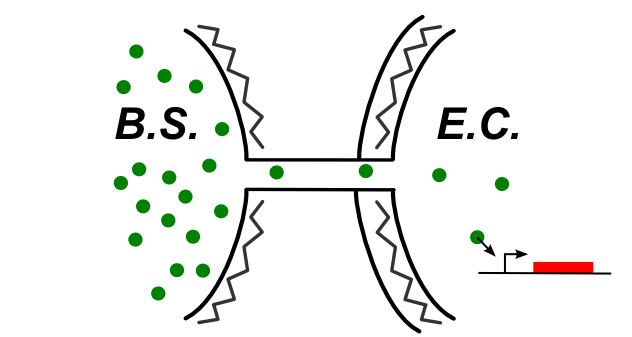 [[File:cytoplasm_connection_with_coli.jpg|thumb|center|upright=2.0|Schematics of the communication happening directly to the cytoplasm]]
[[File:cytoplasm_connection_with_coli.jpg|thumb|center|upright=2.0|Schematics of the communication happening directly to the cytoplasm]]
The compatibility of the different systems will have to verified.
On the other hand, we can re-use their Push-on push-off system of Pekin iGEM team of 2010, adapt it and use it in the receptor cell. The designs are the following:
- [[Team:Paris_Bettencourt/C1_diffusion|The C1(ind) diffusion:]] The indestructible-C1 diffusion will change the toggle switch state
- [[Team:Paris_Bettencourt/RecA_diffusion|The RecA* diffusion:]] The RecA diffusion will help change the toggle switch state
If communication happens with the periplasm
In case the communication happens with the periplasm, we have to think about molecules that can be transported into the cytoplasm.
 [[File:Periplasm_conection.jpg|thumb|center|upright=2.0|Schematic of the communication happening through the periplasm. A special transporter have to be included in the signalling process]]
[[File:Periplasm_conection.jpg|thumb|center|upright=2.0|Schematic of the communication happening through the periplasm. A special transporter have to be included in the signalling process]]
We have to think about more sophisticated approaches. The designs are as following:
- [[Team:Paris_Bettencourt/MPB_diffusion|The MBP diffusion:]] We need a CRP+, MBP- E.Coli mutant. We produce the MBP protein in Bacillus subtilis and make it diffuse through the nanotubes. As long as the MBP has not reached the E. Coli periplasm, the cell cannot digest the maltose in the medium. The indirect induction of MalR by MBP triggers the expression of the GFP reporter.
- [[Team:Paris_Bettencourt/OmpR diffusion|The OmpR diffusion:]] We need a OmpR- Receptor* E. Coli mutant. We produce the OmpR protein in Bacillus Subtilis. As long as the OmpR has not diffused from B. Subtilis, the signaling cascade cannot be activated. With the rescue by Bacillus Subtilis of the OmpR protein, the expression of the reporter gene is activated.
What can we conclude?
If one of these designs works, we would prove that the interspecial nanotube communication exists and would find the eventual location of the connection with the membrane.
Designs for a Master-Slave system
The principle of Step 2 is to build a Master-Slave system, where the Master controls the state of the Slave cell in a monodirectional exchange.
 [[File:Master_Slave_system_principle.jpeg|thumb|center|upright=2.0|Summary of the principle of the Master-Slave experiments]]
[[File:Master_Slave_system_principle.jpeg|thumb|center|upright=2.0|Summary of the principle of the Master-Slave experiments]]
Such a design implies the reversibility of all the sub-systems, the activators and the amplifiers in particular.
Several potential designs are summed-up below:
- [[Team:Paris_Bettencourt/PonPoff_system|The diffusive RecA push-on push-off system:]] In this design we re-use the 2010 Pekin iGEM team's push-on push-off system, but instead of triggering the change by UV, we trigger it with a constitutively active RecA mutant that we diffuse through the nanotubes. We want the emitter cell (the master cell) to control the toggle switch state in the slave cell.
- [[Team:Paris_Bettencourt/ComS_diffusion|The ComS system:]] The caracterisation step of the MeKS system turn to be reversible. It can be considered as well as a Master/Slave system.
Designs for a bidirectional communication
If we succeed in establishing a monodirectional communication, we may go on and try to build a bidirectional communication system. Here is the general draft:
 [[File:Principle_of_bidirectional_communication.jpg|thumb|center|upright=2.0|Summary of the principle of the bidirectional cummunication]]
[[File:Principle_of_bidirectional_communication.jpg|thumb|center|upright=2.0|Summary of the principle of the bidirectional cummunication]]
The genetic design combines the two previously described amplification systems. The complete sum-up is the following:
 [[File:General_Scheme_for_bidirectional_communication.jpg|thumb|center|upright=3.0|Complete design]]
[[File:General_Scheme_for_bidirectional_communication.jpg|thumb|center|upright=3.0|Complete design]]
 "
"
