Team:UTP-Panama/After Regional Week 2
From 2011.igem.org
(→October 24, 2011) |
(→October 25, 2011) |
||
| Line 67: | Line 67: | ||
<div align="center">PURIFICATION PROTOCOL<br> | <div align="center">PURIFICATION PROTOCOL<br> | ||
[[File:Purifprot.png]]</div> | [[File:Purifprot.png]]</div> | ||
| - | + | Source: | |
QIAEX II® Handbook. For DNA extraction from agarose and polyacrylamide gels and for desalting and concentrating DNA from solutions. October 2008. QIAGEN Sample and Assay Technologies. | QIAEX II® Handbook. For DNA extraction from agarose and polyacrylamide gels and for desalting and concentrating DNA from solutions. October 2008. QIAGEN Sample and Assay Technologies. | ||
Revision as of 03:13, 29 October 2011
|
Home |
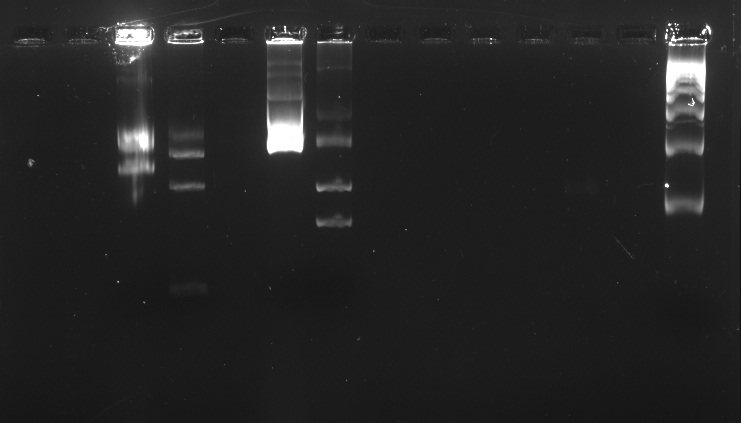
Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | Week 16 | Week 17 | After Regional Week 1 | After Regional Week 2 | Octuber 24 to 28October 24, 2011Morning October 25, 2011We ran an electrophoresis before we started the purification. The bands we needed were the ones with the weight mentioned.
Electrophoresis with BBa_K410000, BBa_K381001, and pSB1A3 digested and undigested In the well labeled as "B" of the picture there is nothing observed because the amount of sample put was very little. Purification Source: QIAEX II® Handbook. For DNA extraction from agarose and polyacrylamide gels and for desalting and concentrating DNA from solutions. October 2008. QIAGEN Sample and Assay Technologies. |
 "
"