Team:UNICAMP-EMSE Brazil/Project
From 2011.igem.org
Neshich.iap (Talk | contribs) |
|||
| Line 52: | Line 52: | ||
EHEC senses two classes of signals to activate its virulence genes, the first is the bacterial aromatic autoinducer (AI-3) produced by the normal GI flora, and the second is the catecholamine class of hormones and in particular epinephrine/norepinephrine produced by the host (Sperandio et al., 2003). AI-3 and epinephrine/norepinephrine are agonists, and the response to both signals can be blocked by α and β-adrenergic antagonists (Sperandio et al., 2003). Both epinephrine and norepinephrine are present in the GI tract. Norepinephrine is synthesized within the adrenergic neurons present in the enteric nervous system (Furness, 2000). Although epinephrine is not synthesized in the enteric nervous system, being synthesized in the central nervous system and in the adrenal medulla, it acts in a systemic manner after being released by the adrenal medulla into the bloodstream, thereby reaching the intestine (Purves et al., 2001). | EHEC senses two classes of signals to activate its virulence genes, the first is the bacterial aromatic autoinducer (AI-3) produced by the normal GI flora, and the second is the catecholamine class of hormones and in particular epinephrine/norepinephrine produced by the host (Sperandio et al., 2003). AI-3 and epinephrine/norepinephrine are agonists, and the response to both signals can be blocked by α and β-adrenergic antagonists (Sperandio et al., 2003). Both epinephrine and norepinephrine are present in the GI tract. Norepinephrine is synthesized within the adrenergic neurons present in the enteric nervous system (Furness, 2000). Although epinephrine is not synthesized in the enteric nervous system, being synthesized in the central nervous system and in the adrenal medulla, it acts in a systemic manner after being released by the adrenal medulla into the bloodstream, thereby reaching the intestine (Purves et al., 2001). | ||
| - | The virulence and motility genes (associated to flagelar proteins) are activated through the AI-3/epinephrine/norepinephrine signaling mechanism (Sperandio et al., 1999; Sperandio et al., 2001; Sperandio et al., 2003). These signals are sensed by sensor kinases such as QseC in the membrane of EHEC and othe pathogens. | + | The virulence and motility genes (associated to flagelar proteins) are activated through the AI-3/epinephrine/norepinephrine signaling mechanism (Sperandio et al., 1999; Sperandio et al., 2001; Sperandio et al., 2003). These signals are sensed by sensor kinases such as QseC in the membrane of EHEC and othe pathogens. That relay this information through a complex regulatory cascade that activates the flagella regulon, and the LEE pathogenicity island, necessary for intestinal colonization through the formation of AE lesions. The sensor for the flagella regulon is QseC that autophosphorylates in response to both epinephrine and AI-3, transfers its phosphate to the QseB response regulator, which in turn activates transcription of the flagella genes through binding at flhDC promoter and itself (Sperandio et al., 2002 Clarke et al, 2005 and Clarke et al, 2006). The AI-3/epinephrine/norepinephrine signaling cascade is present in several bacterial species (e.g. Shigella, Salmonella, Erwinia carotovora, Pasteurella multocida, Haemophilus influenzae, Actinobacillus pleuropneumoniae, Chromobacterium violaceum, Coxiella burnetti, Yersinia, Francisella tularensis and Ralstonia solacearum) suggesting that this interkingdom cross-signaling is not restricted to E. coli. |
== '''Project Description''' == | == '''Project Description''' == | ||
Revision as of 15:09, 28 September 2011

| Home | Project | Methods | Results | Data | Team | Notebook | Human Practices | Safety | Profile | Sponsors | Wix |

Contents |
Abstract
The human immune system comprises a wide range of cells and mechanisms finely regulated to maintain body's homeostasis against eventual threads. However, pathological conditions, such as stress and autoimmune diseases, can cause imbalances in its action rendering higher susceptibility to potential pathogens. This imbalance is ultimately observed in the biased differentiation of naive T-CD4+ Lymphocytes towards T-lymphocyte "helper" class 1 (Th1), in autoimmune diseases, or 2 (Th2), in stressful condition, favoring cellular or humoral adaptive responses, respectively. This pathological imbalance renders the organism more susceptible to the onset of diseases caused by microorganisms. Thus, a mechanism that counteracts this imbalance is highly desirable. The aim of our project is to use the ability of some microorganisms to sense changes in the host´s body homeostasis, used by them as a signal to trigger expression of virulence factors, to counteract this pathological imbalance. In this sense, the ability of some gut-inhabiting bacteria to sense cathecolamines, which can reach high levels on the gastro-intestinal tract during stress, will be used to trigger the production of IL-12, a potent activator of Th1, to counteract the preferential differentiation towards Th2. On the other hand, the ability to sense Nitric-Oxide (NO), a compound released at higher levels in inflammatory conditions, will be used to trigger the production of IL-10, a cytokine with a potent anti-inflammatory action. To switch between both responses, a mechanism that can break down the opposite signaling molecule on the recognition of the first signal will be developed. The microorganism bearing these devices will thus be able to restore the appropriate balance between Th1 and Th2 cells in the host's organism, thus counteracting harmful outcomes of this condition and, hence, improving human life quality.
Background
In this section we describe a background we believe is important to understand the relation between stress and immune system. The main reference used to this description is the review of [http://www.ncbi.nlm.nih.gov/pubmed/10511695 Elenkov and Chorusos (1999)].
The neuroendocrine and immune systems play major roles in adaptation. In general, stress has been regarded as immunosuppressive. Recent evidence, indicates that acute, subacute or chronic stress might suppress cellular immunity and boost humoral immunity. This is mediated by a differential effect of stress hormones, the glucocorticoids and catecholamines (such as adrenaline and norepinephrine), on T helper 1 (Th1)/Th2 cells and type 1/type 2 cytokine production. Acute stress might induce pro-inflammatory activities in certain tissues through neural activation of the peripheral corticotropin-releasing hormone–mast cell–histamine axis. Through these mechanisms, stress might influence the onset and/or course of infectious, autoimmune/inflammatory, allergic and neoplastic diseases Elenkov and Chorusos (1999).
Immune system
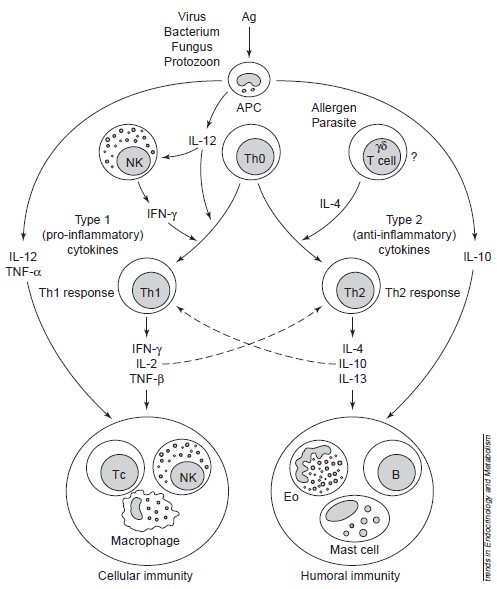
(from Elenkov et al. 1999) To a better description, we must provide a review about immune system. Immune responses are regulated firstly by antigen-presenting cells (APCs), such as the macrophages and dendritic cells (which are components of innate immunity), and by the T helper (Th) lymphocyte subclasses Th1 and Th2, which are components of acquired (adaptive) immunity (see Figure 1). The main secreted cytokines of Th1 cells are [http://en.wikipedia.org/wiki/Interferon-gamma IFN-γ], [http://en.wikipedia.org/wiki/Interleukin_2 IL-2] and [http://en.wikipedia.org/wiki/Transforming_growth_factor_beta TNF-β], which promote cellular immunity, whereas Th2 cells secrete a different set of cytokines, primarily [http://en.wikipedia.org/wiki/Interleukin_4 IL-4], [http://en.wikipedia.org/wiki/Interleukin_10 IL-10] and [http://en.wikipedia.org/wiki/Interleukin_13 IL-13], which promote humoral immunity. IL-10 also displays potent abilities to suppress the antigen presentation capacity of antigen presenting cells. (See Figure 1)
Naive CD4+ Th0 cells are clearly bipotential and serve as precursors of Th1 and Th2 cells. Among the factors currently known to influence the differentiation of these cells towards the Th1 or Th2 subsets, the cytokines produced by cells of the innate immune system are the most important. Thus, IL-12[http://en.wikipedia.org/wiki/Interleukin_12], produced by activated macrophages or other APCs, is the major inducer of Th1 differentiation and hence cellular immunity; this cytokine acts in concert with natural killer (NK)-cell-derived IFN-γ to promote Th1 responses. APC-derived IL-12 and TNF-α, in concert with NK- and Th1-cell-derived IFN-γ stimulate the functional activity of T cytotoxic (Tc) cells, NK cells and activated macrophages, which constitute the major components of cellular immunity. All three cytokines – IL-12, TNF-α and IFN-γ – also stimulate the synthesis of nitric oxide (NO) and other inflammatory mediators that drive chronic delayed-type inflammatory responses. Because of these crucial and synergistic roles in inflammation, IL-12, TNF-α and IFN-γ are considered the major pro-inflammatory cytokines (See Figure 1).
Th1 and Th2 responses are mutually inhibitory. Thus, IL-12 and IFN-γ inhibit Th2, and IL-4 and IL-10 inhibit Th1 responses. IL-4 and IL-10 promote humoral immunity by stimulating the growth and activation of mast cells and eosinophils, the differentiation of B cells into antibody-secreting B cells and B-cell immunoglobulin switching to IgE. Importantly, these cytokines inhibit macrophage activation, T-cell proliferation and the production of pro-inflammatory cytokines. Thus, IL-4 and IL-10 are the major anti-inflammatory cytokines (See Figure 1).
Figure 1: Role of APCs, Th1 and Th2 cells, and proinflammatory and antiinflammatory cytokines in the regulation of cellular and humoral immunity. Cellular immunity provides protection against intracellular bacteria, protozoa, fungi, and several viruses, whereas humoral immunity provides protection against multicellular parasites, extracellular bacteria, some viruses, soluble toxins, and allergens. Solid lines represent stimulation; dashed lines represent inhibition. ABBREVIATIONS: Ag, antigen; APC, antigenpresenting cell; NK, natural killer cell; B, B cell; Th, T helper cell; Tc, T cytotoxic cell; Eo, eosinophil; IL, interleukin; TNF, tumor necrosis factor; IFN, interferon. (From Elenkov and Chrousos, 1999)
Stress and Immunity
Glucocorticoids
The stress hormones (Glucocorticoids and Catecholamines) suppress cellular (Th1 response) and potentiate humoral immunity (Th2 response). Glucocordicoids suppress the production of TNF-α, IFN-γ and IL-2 in vitro and in vivo in animals and humans (Chorousos et al 1995). Additionally, these hormones act through their classic cytoplasmic and nuclear receptors on APCs to suppress the production of the main inducer of Th1 responses, IL-12, in vitro and ex vivo. Since IL-12 is extremely potent in enhancing IFN-γ and inhibiting IL-4 synthesis by T cells, the inhibition of IL-12 production might be a major mechanism by which glucocorticoids affect the Th1/Th2 balance (Elenkov et al 1996). The same treatment of monocytes/macrophages is also associated with an increased production of IL-4 by T cells, probably as a result of blocking the suppressive effects of IL-12 on Th2 activity.
Catecholamines
CAs drive a Th2 shift, both at the level of APCs and Th1 cells. Elenkov et al (1996) demonstrated recently that NA and adrenaline potently inhibited or enhanced the production of IL-12 and IL-10, respectively in human whole blood cultures stimulated with lipopolysaccharide (LPS) ex vivo. These effects are mediated by stimulation of β-adrenoceptors (ARs), as they are completely prevented by propranolol, a β-AR antagonist. The non-selective β-AR agonists and selective β2-AR agonists inhibited the production of IL-12 in vitro and in vivo as it is shown by Panina-Bordignon et al (1997). In conjunction with their ability to suppress IL-12 production, β2-AR agonists inhibited the development of Th1-type cells, while promoting Th2 cell differentiation. This effect occurs because β2-ARs are expressed on Th1 cells, but not on Th2 cells (Sanders et al 1997). In fact, in both murine and human systems, β2-AR agonists inhibit IFN-γ production by Th1 cells, but do not affect IL-4 production by Th2 cells (Sanders et al 1997).
Bachen et al (1995) administrated a nonselective adrenoceptor antagonist (labetalol) to subjects exposed to mental stress. The inactivation of adrenergic signaling diminished ratio of CD4:CD8 cells after the stressor, which can reinforce that there is a direct evidence of sympathetic nervous system influence to immune system.
Importantly, the differential effect of CAs on type 1/type 2 cytokine production also operates in vivo. Thus, increasing sympathetic outflow in mice by selective α2-AR antagonists or application of β-AR agonists results in inhibition of LPS-induced TNF-α and IL-12 production (Elenkov et al 1995b). Maybe this effect can improve susceptibility to LPS-containing pathogens (since they will not induce the activity of immune system to react to their presence).
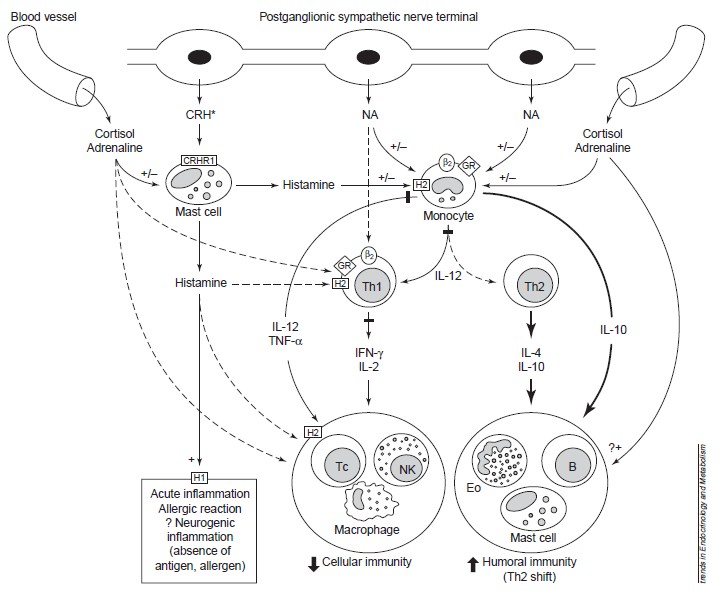
The figure below can show more details about these processes.
Figure 2: Stress and CRH influence immune/inflammatory and allergic responses by stimulating glucocorticoid, catecholamines and peripheral (immune) CRH secretion and by altering the production of key regulatory cytokines and histamine. *CRH is also released from sensory nerves upon their activation. Solid lines represent stimulation, heavy solid lines represent increased stimulation and dashed lines represent inhibition. Abbreviations: b2, b-2-adrenoceptor; +/–, stimulation/inhibition; B, B cell; CRH, peripheral (immune) corticotropin-releasing hormone; CRHR1, CRH receptor 1; Eo, eosinophil; GR, glucocorticoid receptor; H1/H2, histamine 1/2 receptors; IFN interferon. (From Elenkov and Chrousos, 1999).
The AI-3/epinephrine/norepinephrine signaling system
(from Walters and Sperandio, 2006) Recently, Sperandio et al (1999, 2001 and 2002) created a real revolution in microbiology. They discovered that enteropathogenic bacteria such as enterohemorrhagic E. coli (EHEC) can establish an interkingdon communication system, in which bacteria can sense host hormone levels (such as catecholamines) and respond by altering gene expression and multiplication (Sperandio et al, 2003). EHEC O157:H7 is responsible for major outbreaks of bloody diarrhoea and hemolytic uraemic syndrome (HUS) throughout the world. EHEC has a very low infectious dose (as few as 50 cfu), which is one of the major contributing factors to EHEC outbreaks. EHEC colonizes the large intestine where it causes attaching and effacing (AE) lesions. The AE lesion is characterized by the destruction of the microvilli and the rearrangement of the cytoskeleton to form a pedestal-like structure, which cups the bacteria individually.
EHEC senses two classes of signals to activate its virulence genes, the first is the bacterial aromatic autoinducer (AI-3) produced by the normal GI flora, and the second is the catecholamine class of hormones and in particular epinephrine/norepinephrine produced by the host (Sperandio et al., 2003). AI-3 and epinephrine/norepinephrine are agonists, and the response to both signals can be blocked by α and β-adrenergic antagonists (Sperandio et al., 2003). Both epinephrine and norepinephrine are present in the GI tract. Norepinephrine is synthesized within the adrenergic neurons present in the enteric nervous system (Furness, 2000). Although epinephrine is not synthesized in the enteric nervous system, being synthesized in the central nervous system and in the adrenal medulla, it acts in a systemic manner after being released by the adrenal medulla into the bloodstream, thereby reaching the intestine (Purves et al., 2001).
The virulence and motility genes (associated to flagelar proteins) are activated through the AI-3/epinephrine/norepinephrine signaling mechanism (Sperandio et al., 1999; Sperandio et al., 2001; Sperandio et al., 2003). These signals are sensed by sensor kinases such as QseC in the membrane of EHEC and othe pathogens. That relay this information through a complex regulatory cascade that activates the flagella regulon, and the LEE pathogenicity island, necessary for intestinal colonization through the formation of AE lesions. The sensor for the flagella regulon is QseC that autophosphorylates in response to both epinephrine and AI-3, transfers its phosphate to the QseB response regulator, which in turn activates transcription of the flagella genes through binding at flhDC promoter and itself (Sperandio et al., 2002 Clarke et al, 2005 and Clarke et al, 2006). The AI-3/epinephrine/norepinephrine signaling cascade is present in several bacterial species (e.g. Shigella, Salmonella, Erwinia carotovora, Pasteurella multocida, Haemophilus influenzae, Actinobacillus pleuropneumoniae, Chromobacterium violaceum, Coxiella burnetti, Yersinia, Francisella tularensis and Ralstonia solacearum) suggesting that this interkingdom cross-signaling is not restricted to E. coli.
Project Description
Stress can be understood as the perception of a potentially dangerous state for homeostasis maintenance, triggering a series of physiological responses. Any stressor or threat to stability of the organism is counteracted by adaptive responses. The effectors that trigger the physiological responses to stressful conditions are the corticotropin-release hormone (CRH) and the locus ceruleus-noradrenaline (LC-NA)/sympathetic neurons of the hypothalamus. The activity of these neurons regulate the activation of HPA (Hypothalamic-Pituitary-Adrenal) axis and the Sympathetic Nervous System that result in systemic elevations in levels of glucocorticoids (GCs) and cathecolamines (CAs), like epinephrine and norepinephrine.
There has been convincing evidence that the GCs and CAs at the high levels achieved in stress can influence the immune response in an important way, acting as an immune suppressor agent of cellular immunity (Elenkov et al., 1999). Specially, CAs shift differentiation of naive T CD4+ Lymphocytes into the Th2 (T-lymphocyte "helper" class 2) lineage, via inhibition of the production of the cytokine IL-12 (Interleukin 12, a potent activator of differentiation into Th1 Lymphocytes lineage) and induction of the production of the cytokine IL-10, as was shown in a study using human blood cultures stimulated with lipopolysaccharide (LPS) ex vivo (Elenkov et al., 1996). This effect can be completely prevented using propanolol, an antagonist of β-Adrenoceptors. Together with GCs, CAs lead to a suppression of antigen-presenting cells (APCs) and Th1 cells. The latter mediates the cellular immune response. This polarization affects the immune response, causing imbalance to an increased susceptibility to disease and immunosuppression.
Recently, it was demonstrated that several bacterial pathogens (such as Escherichia, Yersinia and Pseudomonas) can sense host cues and so activate expression of their virulence genes. In the case of host stress, bacteria can sense the signal that defines a susceptible condition of the host immune system (characterized by high levels of CAs and GCs). In other words, such pathogens can sense and respond to increasing levels of CAs as a way to recognize the host environment and to promote the expression of virulence and growth factors, via a Bacterial Adrenergic Receptor protein, QseC, and its signaling pathway (Reading et al., 2009). The control of this bacterial system is supposed to be a new way to prevent diseases.
The use of probiotics has been explored extensively so far, however, there is an increasing pressure for improvements in the formulation of its composition. The use of genetic engineering and rational design of genetically modified organisms (GMOs) has been considered as an alternative against onset of stress and diseases. For example, Steidler et al. (2000) used a genetically modified strain of Lactococcus lactis expressing the gene for interleukin IL-10 to treat mice presenting irritable bowel syndrome (IBD), by means of bacteria being injected directly into the gastric lumen of mice.
Our project aims to produce a probiotic capable of restoring the balance between Th1 and Th2 immune response, with the JEDI BACTERIA, in order to improve the quality of life and reduce the effects triggered by stress, such as induction of virulence genes of Bacterial Pathogens. Through expression and secretion of IL12 and degradation of NO under high levels of Cathecolamines, we hope we would be able to induce Th1 lineage of Lymphocytes and suppress Th2, declaring a great 'War' against the Stress.
Complete description of devices
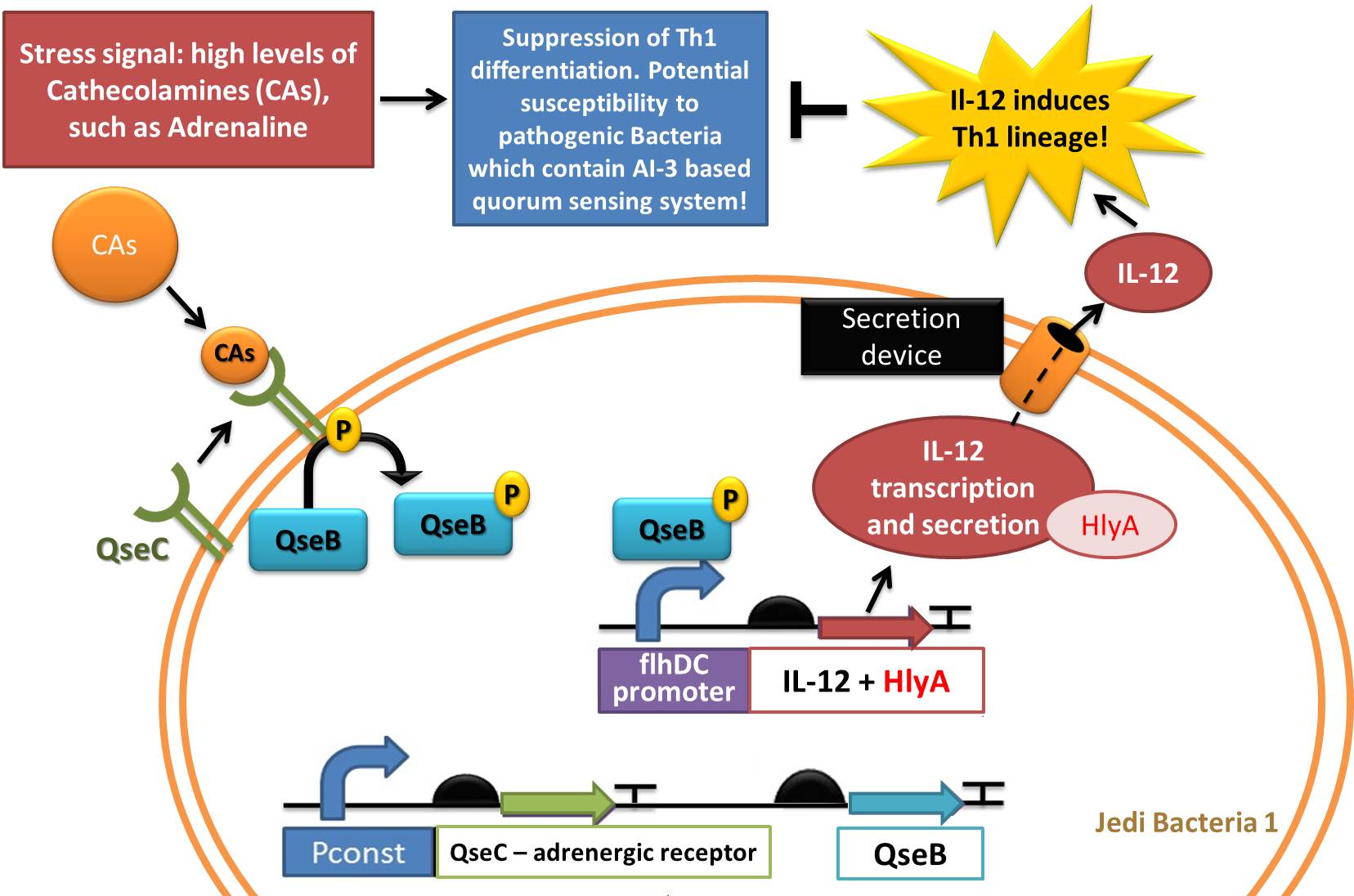
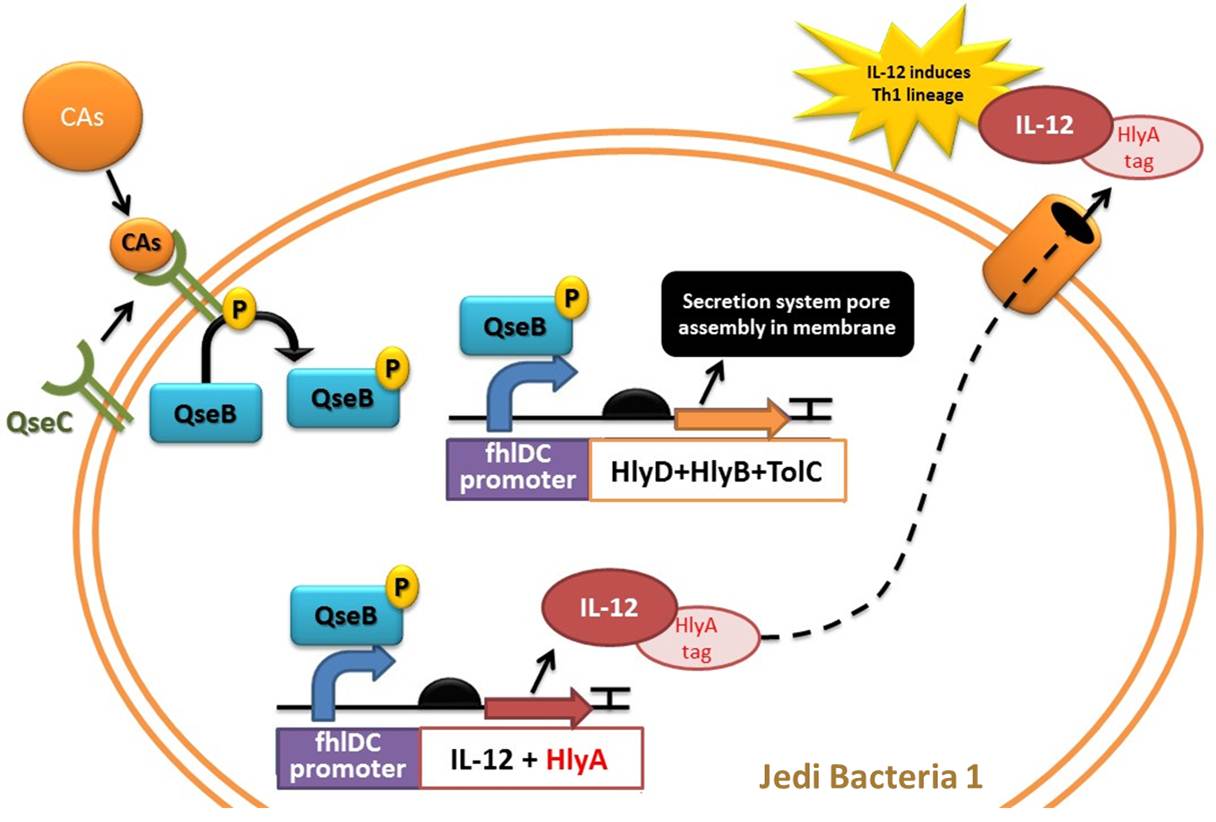
Device 1: Adrenaline sensor/IL-12 producer
Figure 3: Representation of Jedi bacteria containing device 1 (able to sense high levels of CAs and respond by producing IL-12) and device 3 (secretion system device, represented as the orange cylinder).
Figure 4: Another representation of device 1 (able to sense high levels of CAs and respond by producing IL-12) and device 3 (secretion system device).
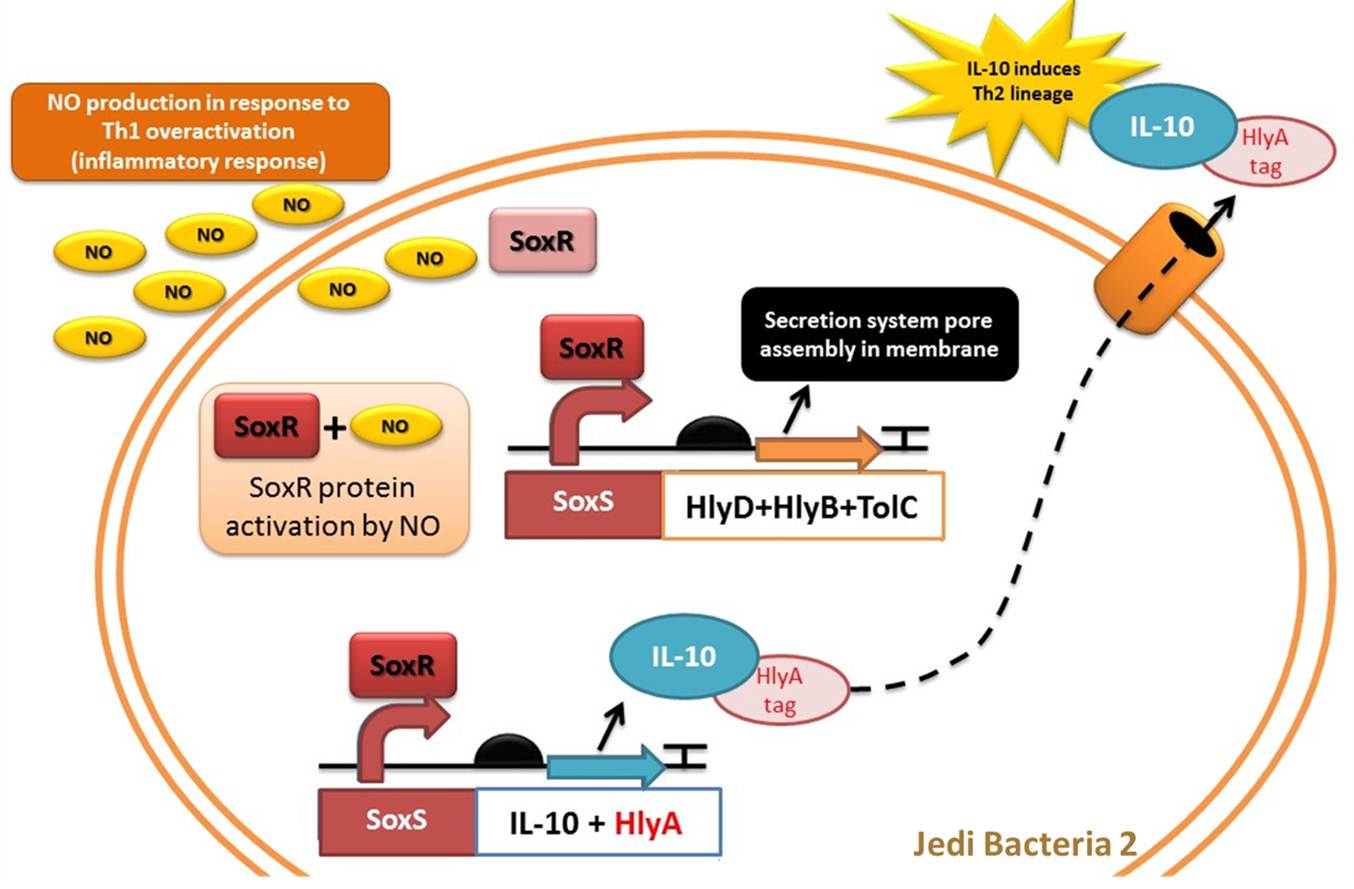
Device 2: NO sensor/IL-10 producer
This device is a modification of Stanford team anti-inflammatory device (iGEM 2009 - https://2009.igem.org/Team:Stanford/ProjectPage), which comprises SoxR gene (BBa_K223047) under control of a Constitutive Promoter (BBa_J23119) and SoxS promoter (BBa_K223001 – deleted part), coupled to human IL-10 gene (sequence designed by the team, improved for bacterial expression).
It acts by recognizing an inflammatory signal (NO - nitric oxide) coupled to IL-10 transcription activation. The team chooses IL-10 to impair inflammatory response mediated by Th1 overstimulation (due to Device 1 action), to reach an equilibrium between immune stimulation (Th1) and suppression (Th2).
Nitric oxide is generated by immune cells (monocytes, macrophages and neutrophils) as a part of immune response, since it as signaling molecule and is toxic to bacterial pathogens. These cells possess an inducible nitric oxide synthase (iNOS), which is activated by cytokines as tumor necrosis factor (TNF) and interferon-gamma (IFN-γ) released by macrophages and regulatory T-cells (Hibbs et al 1988; Wink et al 1991).
IL-10 is an anti-inflammatory interleukin, produced mainly by monocytes. It is responsible for down-regulating the synthesis of pro-inflammatory cytokines like IFN-γ, IL-2, IL-3, TNFα (Th1 cytokines), class II MHC, and co-stimulatory molecules on macrophages. IL-10 also displays potent abilities to suppress the antigen presentation capacity of APCs (Antigen Presenting Cells), and it is also responsible for enhancing B cell survival, proliferation and antibody secretion. In this scenario IL-10 favors Th2 response (Mocellin et al 2004, Elenkov & Chrousos, 1999).
Some studies suggested that IL-10 acts as immunoregulator in intestinal tract. For example Steidler et al (2000) used a genetically modified Lactococcus lacti expressing IL-10 to treat mice with Inflammatory Bowel Disease (IBD), and the inflammatory response signals were reduced in 50%. IBD is an autoimmune disease which, unlike the immune response found in intestinal tract due to stress, involves a Th1 polarization and inflammation.
As NO is toxic to bacteria, many bacterial pathogens have evolved mechanisms for nitric oxide resistance. For example, E. coli can naturally respond to redox signals through a superoxide stress system composed by SoxR gene and SoxS promoter. The [http://partsregistry.org/Part:BBa_K554003 SoxR] gene encodes the SoxR transcription factor, which is activated by NO. Thus, in the presence of NO, SoxR activates transcription of the gene regulated by [http://partsregistry.org/Part:BBa_K554000 SoxS promoter] (Hidalgo et al, 1998).
Figure 5: Representation of Jedi bacteria containing device 2 (able to sense high levels of NO and respond by producing IL-10) and device 3 (secretion system device).
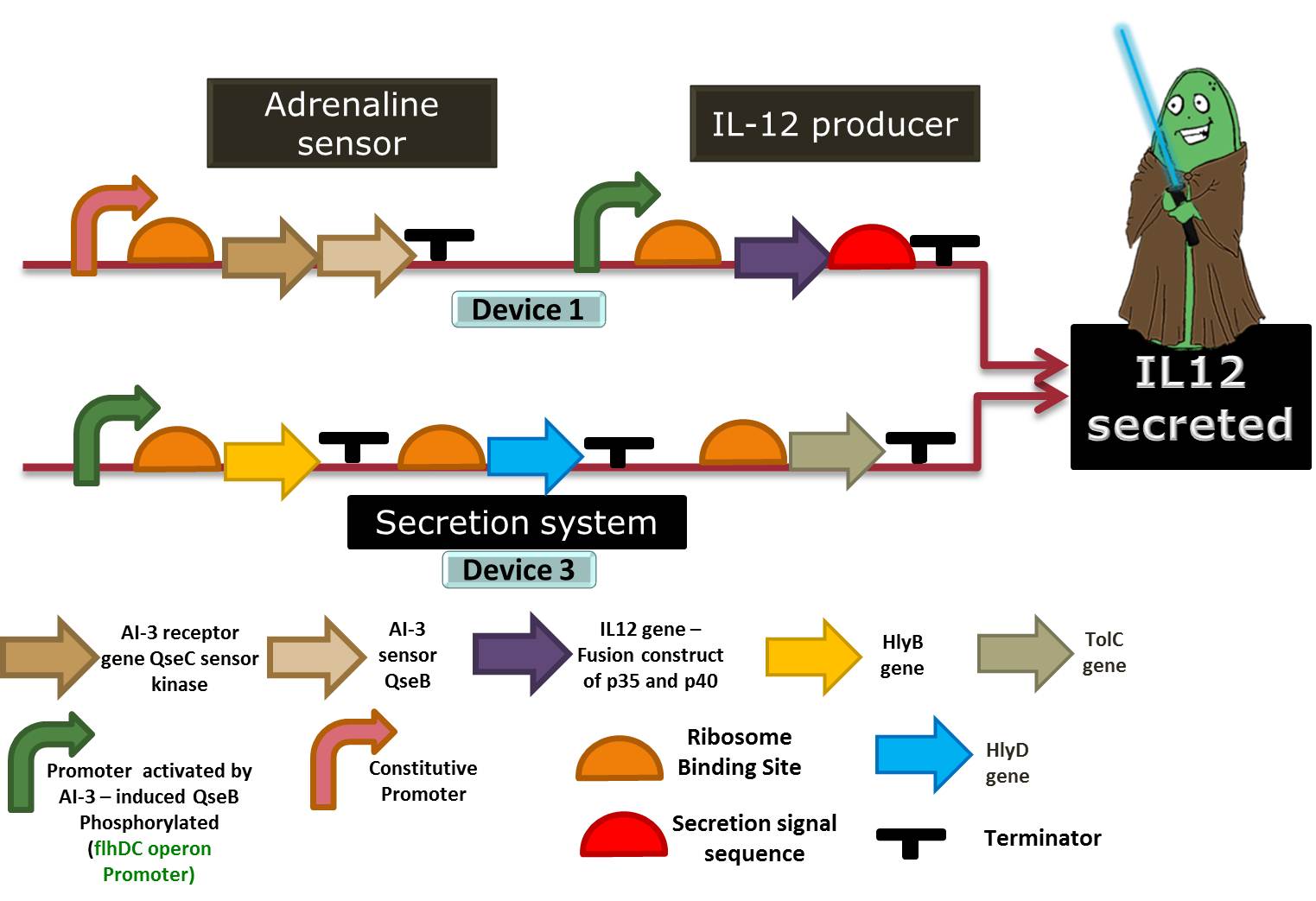
Device 3: Secretion system
So our bacteria have sensed the stress, and have reacted to this by successfully producing interleukins. But there’s no use in storing our interleukins, since they can only act outside the cell, directly on our immune system. Therefore we had to add to our Stress Warrior a system that allows its secretion.
We chose to use the ABC protein-mediated transporter, typical of Gram-negative bacteria, just like our E. coli. It’s composed of 4 parts: the C-terminal signal sequence of alpha-hemolysin (HlyA), the two specific translocator proteins HlyB and HlyD and the outer membrane protein TolC.
HlyB acts as a ATP-binding cassette, and recognizes the substrate via its secretion signal (like HlyA) and is responsible for the specificity of the secretion process. HlyD acts as a membrane fusion or adaptor protein, consisting of a short cytoplasmic domain at the N-terminus followed by a membrane anchor and a large periplasmic domain; it is believed to establish specific links between the outer and the inner membrane components of the system. Finally, TolC is a specific outer membrane protein, which forms a long channel throughout the outer membrane and the periplasm, largely open towards the extracellular medium. These 3 components will be kept expressed in our bacteria at all times.
HlyA, the signal sequence, seems to interact with the cytoplasmic region of the pre-formed HlyB–D complex. After the binding of the HlyA secretion signal by the HlyB–D complex, HlyD induces the interaction with TolC. To be effective, only the last 50-60 aminoacids of the C-terminal of Hly-A are required, so we can use it as a fusion protein that is attached to the interleukin and allows it to be secreted. Besides, HlyA is itself a weak antigen, turning it harmeless to the immune responses we are trying to interfere with. References: Tzschaschel (1996), Delepelaire (2004) and Gentschev (2002).
You can see a representation of this device acting in the schema below:
Figure 6: Representation of device 3, the protein secretion system, in a Jedi bacteria that contains Device 1 (Adrenaline sensor/IL-12 producer). To export a protein, the bacteria must have the HlyD, HlyB and TolC proteins and the target protein must have a signal sequence (HlyA tail). In this case, the target protein to be secreted is IL-12.
Linker: the Nor sequence
In addition to the sensor, generator and secretion systems, we thought about designing a part which would link the action of device 1 and device 2. Indeed, we have to induce the appropriate switch to Th1 or Th2 differentiation and avoid a simultaneous action of these two devices or some “yo-yo” phenomena. To make this link, we thought about adding to the first device a nitric oxide reductase (nor) sequence. This sequence consists in four genes, norC, norB, norQ and norD, which encode a NO reductase (Nor) complex.
NorC and norB are structural genes. NorC is 444 bp in length and encodes a protein which contains a cytochrome c subunit responsible for transferring electrons to the norB-encoded protein. NorB is 1,341 bp in length and encodes a product which has great similarities with subunit I of cytochrome c oxidases and likely contains the active site. NorQ and norD are respectively 789 bp and 1,869 bp in length and encode for hydrophilic, cytoplasmic proteins which are possibly involved in the Nor complex assembly. The role of these proteins has not been yet elucidated but research show that the four genes, norCBQD, are required for production of an active Nor complex.
The NO reductase complex is responsible for NO reduction and catalyzes the following reaction: 2NO + 2H+ &rarr N2O + H2O. If device 1 is activated by the sensing of adrenaline, the Nor complex will be activated and the precedent reaction will take place, consuming all the surrounding NO molecules. Since device 2 is activated by the sensing of NO, its action will be then inhibited. Thanks to this sequence, we could make sure that when device 1 is working, device 2 cannot be activated in the same time.
We didn’t have the possibility to synthesize this sequence for costs reasons and we couldn’t amplify it by lack of time but it seems very promising. This sequence has never been studied and developed by any iGEM team and we believe it is an interesting suggestion of work for future teams.
The Experiments
Check our device testing experiments at Results page and the day-to-day work done to built the devices at our Notebook page.
Applications
- It’s really well known how stressful is our life and how much we are affected by this. But what is Stress exactly? Stress is a word with a broad meaning, but generally it relates to a set of symptoms and reactions showed by our bodies in response to external factors that bring us pressure, anxiety or any emotional imbalance.
- It is not exactly something easy to control!
- Can you simply stop caring about being fired? About your tests next week? Or can you decide to ignore the iGEM deadlines? Well, we just need to accept that stress is part of our lives and learn how to deal with it.
- Regarding this, our team wanted to develop a way to reduce stress symptoms that directly affect our health, and our focus is the immune system. The major application we expect to get from Stress Wars would be the launching of a stress-beating-probiotic.
- In our view, stress is present in our every day life, so a probiotic, which is consumed on a regular basis, could keep the immune balance. Responding to both adrenaline and NO stress, it would cover the two major sources of imbalance, by over expressing interleukins and redirecting the immune response without adding exogenous substances or medication. This could guarantee a long-term immune balance that can only favor health.
- Besides, the idea of affecting the immune balance is really useful and has various potential applications, including the optimization of the response to various vaccines and the treatment of several diseases that are known to be caused by immune imbalances, such as cancer, HIV, type I Diabetes and Multiple Sclerosis.
REFERENCES
Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab. 1999 Nov;10(9):359-368. [http://www.ncbi.nlm.nih.gov/pubmed/10511695 Link to Pubmed ]
Elenkov, I.J., Papanicolaou, D.A., Wilder, R.L. and Chrousos, G.P. (1996) Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc. Assoc. Amer. Physic. 108, 374–381. [http://www.ncbi.nlm.nih.gov/pubmed/8902882 Link to Pubmed ]
Reading NC, Rasko DA, Torres AG, Sperandio V. The two-component system QseEF and the membrane protein QseG link adrenergic and stress sensing to bacterial pathogenesis. Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):5889-94. Epub 2009 Mar 16. [http://www.ncbi.nlm.nih.gov/pubmed/19289831 Link to Pubmed]
Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10, Science 289. 2000. 1352–1355. [http://www.ncbi.nlm.nih.gov/pubmed/10958782 Link to Pubmed]
Barbara D. Tzschaschel, Carlos A. Guzmán,, Kenneth N. Timmis and Victor de Lorenzo. An Escherichia coli hemolysin transport system-based vector for the export of polypeptides: Export of shiga-like toxin IIeB subunit by Salmonella typhimurium aroA. Nature Biotechnology 14, 765 - 769 (1996) [http://www.nature.com/nbt/journal/v14/n6/abs/nbt0696-765.html Article link]
P. Delepelaire. Type I secretion in Gram-negative bacteria. Biochimia et Biophysica Actca 1694, 149-161 (2004) [http://www.ncbi.nlm.nih.gov/pubmed/15546664 Link to PubMed]
Ivaylo Gentschev, Guido Dietrich and Werner Goebel. The E. coli α-hemolysin secretion system and its use in vaccine development. Trends in Microbiology 10, 39-45 (2002) [www.ncbi.nlm.nih.gov/pubmed/11755084 Link to PubMed]
Bachen EA, Manuck SB, Cohen S, Muldoon MF, Raible R, Herbert TB, Rabin BS.Adrenergic blockade ameliorates cellular immune responses to mental stress in humans. Psychosom Med. 1995 Jul-Aug;57(4):366-72. [http://www.ncbi.nlm.nih.gov/pubmed/?term=Adrenergic%20Blockade%20Ameliorates%20Cellular%20Immune%20Responses%20%20to%20Mental%20Stress%20in%20Humans Link to PubMed]
Chrousos G.P., The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. New Engl. J. Med., 332 (1995), pp. 1351–1362. [http://www.ncbi.nlm.nih.gov/pubmed/7715646 Link to PubMed]
I.J. Elenkov, D.A. Papanicolaou, R.L. Wilder and G.P. Chrousos, Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc. Assoc. Amer. Physic., 108 (1996), pp. 374–381.
P Panina-Bordignon, et al. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J. Clin. Invest., 100 (1997), pp. 1513–1519.
V.M. Sanders, R.A. Baker, D.S. Ramer-Quinn, D.J. Kasprowicz, B.A. Fuchs and N.E. Street, Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J. Immunol., 158 (1997), pp. 4200–4210.
I.J. Elenkov, G. Hasko, K.J. Kovacs and E.S. Vizi, Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha production by selective alpha- and beta-adrenergic drugs in mice. J. Neuroimmunol., 61 (1995b), pp. 123–131.
 "
"