Team:UNICAMP-EMSE Brazil/Results
From 2011.igem.org
Neshich.iap (Talk | contribs) |
Neshich.iap (Talk | contribs) (→Functional tests) |
||
| Line 13: | Line 13: | ||
===Functional tests=== | ===Functional tests=== | ||
The students assembled the devices subparts: the SoxR/SoxS sensor ([https://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_2:_NO_sensor.2FIL-10_producer Device 2, NO sensor device/ IL-10 producer]), the Adrenaline sensor ([https://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_1:_Adrenaline_sensor.2FIL-12_producer Device 1, CA/AI-3 sensor device/ IL-12 producer]), and the hemolysin secretion system ([https://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_3:_Secretion_system Device 3, Protein Secretion System]). All the sensors were also built with GFP downstream of the sensor itself, replacing the Device products: IL-12 at [https://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_1:_Adrenaline_sensor.2FIL-12_producer Device 1] and IL-10 at [https://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_2:_NO_sensor.2FIL-10_producer Device 2]. | The students assembled the devices subparts: the SoxR/SoxS sensor ([https://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_2:_NO_sensor.2FIL-10_producer Device 2, NO sensor device/ IL-10 producer]), the Adrenaline sensor ([https://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_1:_Adrenaline_sensor.2FIL-12_producer Device 1, CA/AI-3 sensor device/ IL-12 producer]), and the hemolysin secretion system ([https://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_3:_Secretion_system Device 3, Protein Secretion System]). All the sensors were also built with GFP downstream of the sensor itself, replacing the Device products: IL-12 at [https://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_1:_Adrenaline_sensor.2FIL-12_producer Device 1] and IL-10 at [https://2011.igem.org/Team:UNICAMP-EMSE_Brazil/Project#Device_2:_NO_sensor.2FIL-10_producer Device 2]. | ||
| + | |||
| + | <br> | ||
==Device 2 testing: SoxR/SoxS system regulating GFP production== | ==Device 2 testing: SoxR/SoxS system regulating GFP production== | ||
Revision as of 03:45, 27 September 2011

| Home | Project | Methods | Results | Data | Team | Notebook | Human Practices | Safety | Profile | Sponsors | Wix |

Overview
Functional tests
The students assembled the devices subparts: the SoxR/SoxS sensor (Device 2, NO sensor device/ IL-10 producer), the Adrenaline sensor (Device 1, CA/AI-3 sensor device/ IL-12 producer), and the hemolysin secretion system (Device 3, Protein Secretion System). All the sensors were also built with GFP downstream of the sensor itself, replacing the Device products: IL-12 at Device 1 and IL-10 at Device 2.
Device 2 testing: SoxR/SoxS system regulating GFP production
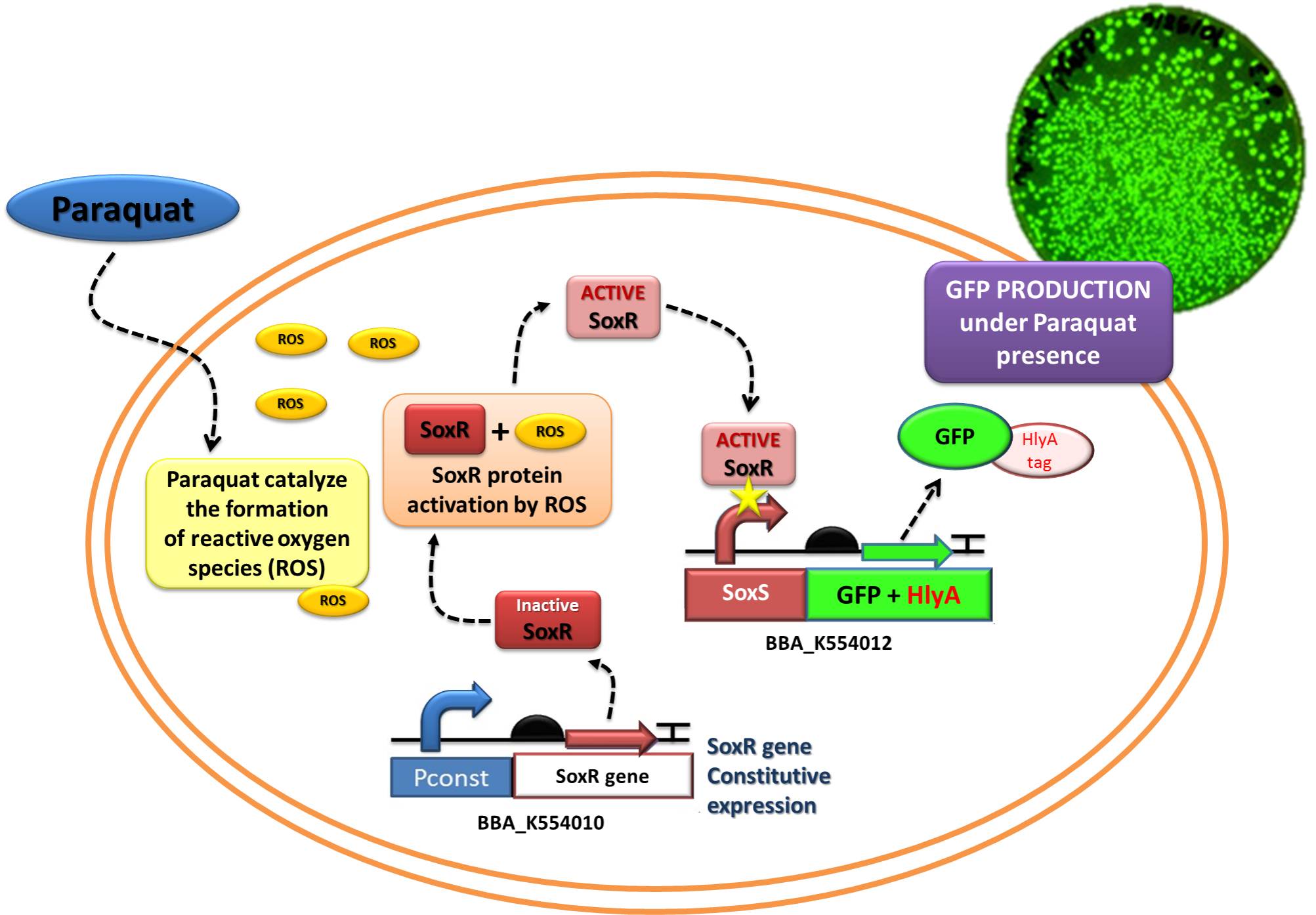
The Device 2 is a modification of Stanford team anti-inflammatory device (iGEM 2009 - https://2009.igem.org/Team:Stanford/ProjectPage), which comprises SoxR gene (BBa_K223047) under control of a Constitutive Promoter (BBa_J23119) and SoxS promoter (BBa_K223001 – deleted part), coupled to human IL-10 gene (sequence designed by the team, improved for bacterial expression).
In order to test the ability of Jedi Bacteria in sensing NO levels and activating genes linked to SoxS promoter, we built a Device testing, with GFP linked to SoxS promoter, as it is shown in the following schema (Figure 1):
The ability to recognize NO (nitric oxide), an inflammation signal molecule, was characterized for SoxR/SoxS sensor, and found to be FUNCTIONAL. In the section below we present the detailed results.
The NO is a product related to inflammatory response in vivo. To test the sensor in vitro, we used http://en.wikipedia.org/wiki/Paraquat Paraquat as inducer, a molecule which imposes superoxide stress within the cell in a similar manner as nitric oxide.
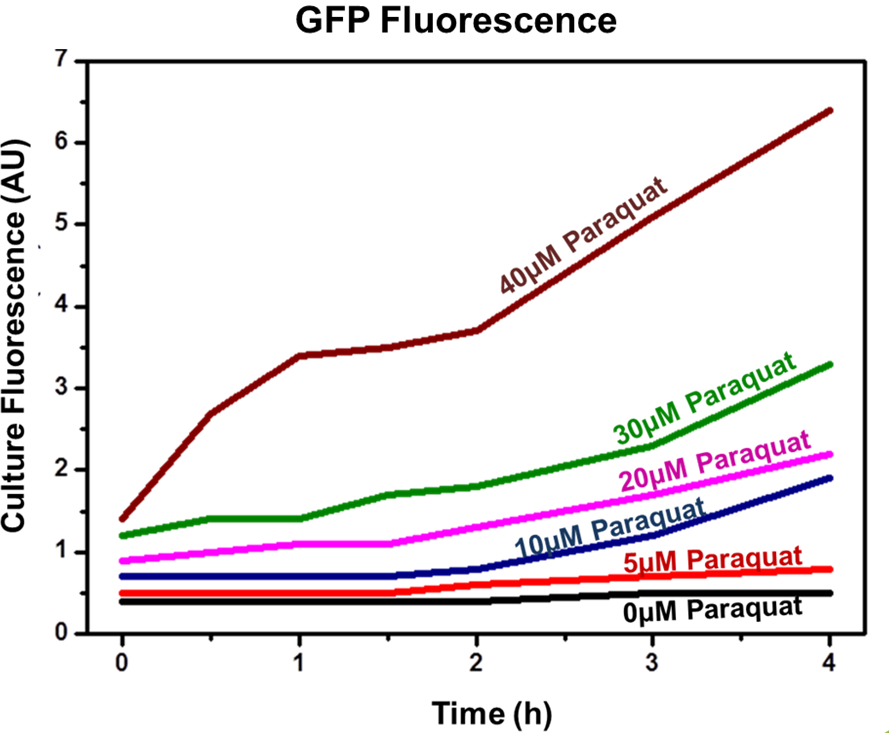
The experiment indicated that our sensor was capable of inducible production of GFP (or another protein controlled by the sensor). In addition, the protein induction can be modulated through varying the inducer concentration (as shown by Figure 2). Higher concentrations of Paraquat exhibited higher fluorescence levels, which indicates increased GFP concentrations.
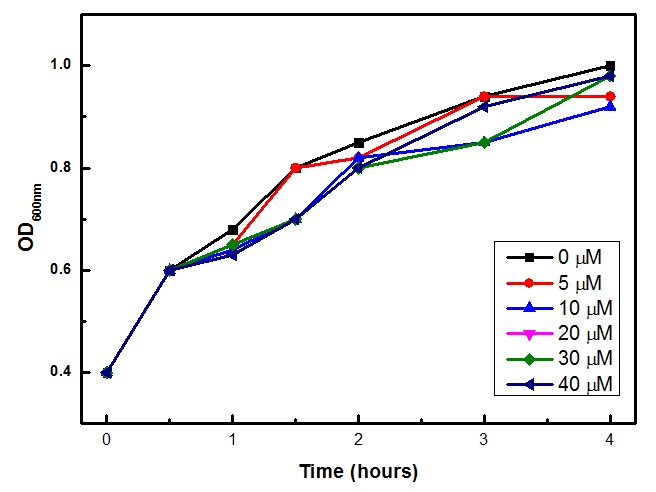
The experimental concentrations of Paraquat did not show significant differences in cell growth as shown by OD levels in Figure 3. The Stanford team (iGEM 2009) showed that Paraquat can be toxic to E. coli cells, with growth inhibition at 60 – 80 µM.

 "
"