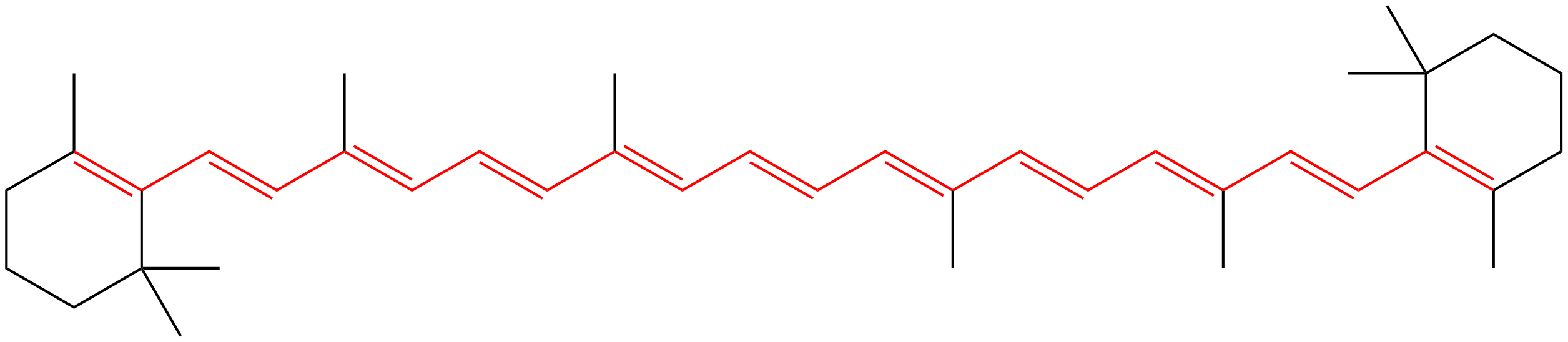
Vitamin A
The metabolite beta-carotene is vitamin A. It is responsible for giving carrots their orange color. Beta-carotene and carotenoids in general are present in many plants and share a common metabolic precursor with other critical compounds including chlorophyll, tocopherol (vitamin E), phylloquinone (vitamin K1), squalene, and other sterols. Humans cannot produce beta-carotene, but we require it as a pigment necessary for color vision.
Metabolic engineering
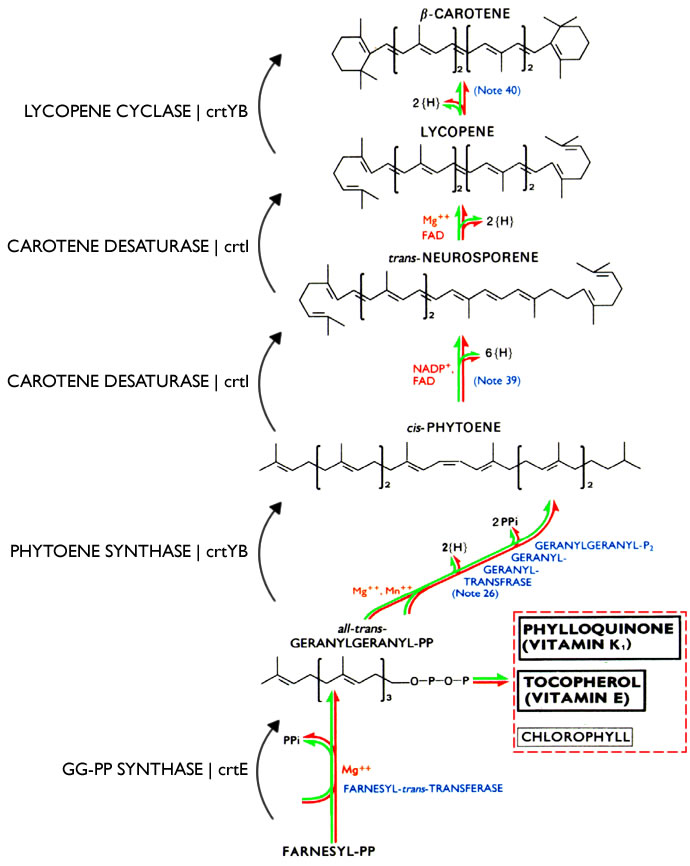
S. cerevisiae has endogenous machinery to produce farnesyl diphosphate, but the rest of the pathway must be engineered. See this schematic for a complete view of the synthetic pathway.
Previous work has been conducted in which the successful implementation of the Beta-carotene producing enzymes have been engineered into S. cerevisiae from carotenogenic genes from X. dendrorhous. This pathway can be viewed here1.
Pathway
The Vitamin A production pathway we used as a template to move into Saccharomyces cerevisiae is from another strain of yeast Xanthophyllomyces dendrorhous which does produce beta carotene. S. cerevisiae has endogenous machinery to produce farnesyl diphosphate, but the rest of the pathway must be engineered. The caretenogenic genes that needed to be moved over were carotene desaturase, GGPP synthase, phytoene synthase. The pathway for its production is as follows:
Once we had the strains with the genes in them on expression cassettes we verified betacarotene production using HPLC analysis. Being sure that the cells were indeed producing betacarotene, we tested its efficiency on a new kind of media, 'dough media'. Dough media is a watered down version of dough with agar. As the final substrate we wanted to use our yeast on was dough we wanted to see how well it performed. We characterized its beta carotene production over time both on YPD plates and on our dough media plates to see what effect the change of substrate would have on the betacarotene yield from the cells.
Sauer, M., Branduardi, P., Valli, M., & Porro, D. (2004). Production of L-ascorbic acid by metabolically engineered Saccharomyces cerevisiae and Zygosaccharomyces bailii. Applied and environmental microbiology, 70(10), 6086-91. doi: 10.1128/AEM.70.10.6086-6091.2004.
Experiments
Vitamin C concentration Assay (By Anne Marie Helmenstine, Ph.D) 1. Add 25.00 ml of vitamin C standard solution to a 125 ml Erlenmeyer flask. 2. Add 10 drops of 1% starch solution. 3. Rinse your buret with a small volume of the iodine solution and then fill it. Record the initial volume. 4. Titrate the solution until the endpoint is reached. This will be when you see the first sign of blue color that persists after 20 seconds of swirling the solution. 5. Record the final volume of iodine solution. The volume that was required is the starting volume minus the final volume. 6. Repeat the titration at least twice more. The results should agree within 0.1 ml. 7. You titrate samples exactly the same as you did your standard. Record the initial and final volume of iodine solution required to produce the color change at the endpoint.
 "
"