Team:Harvard/Technology/Chip Synthesis
From 2011.igem.org
Nidanaushad (Talk | contribs) (→This is a header!) |
Nidanaushad (Talk | contribs) (→Chip Synthesis) |
||
| Line 15: | Line 15: | ||
[[File:HARVChip1.png|center|900 px]] | [[File:HARVChip1.png|center|900 px]] | ||
</div> | </div> | ||
| + | |||
| + | ==Protocol== | ||
| + | ===qPCR of subpools=== | ||
| + | ''Complete protocol found in the supplementary material of Kosuri 2010'' | ||
| + | |||
| + | Perform one qPCR reaction for each single stranded sub-pool, using the following recipe: | ||
| + | *100 ul Sybr Green master mix | ||
| + | *200 nm primer | ||
| + | *2 ul pooled oligo | ||
| + | *Water to bring total volume up to 200 ul | ||
| + | |||
| + | When performing the PCR reaction, one wants to watch the graphs produced by the thermocycler. Initially, the reaction will proceed at an exponential rate. However, as soon as it begins to plateau, the reaction should be stopped. Until this point, oligos were being amplified equally. However, once the plateau is reached, oligos are amplified at unqual rates. It will take approximately 10 cycles to reach the plateau. | ||
| + | |||
| + | On the thermocycler, run the following program: | ||
| + | #1 minute at 95* | ||
| + | #10 seconds at 95* | ||
| + | #30 seconds at the primer annealing temperature | ||
| + | #Go to step 2 until the reaction plateaus. | ||
| + | |||
| + | The PCR product should undergo PCR purification. | ||
| + | |||
| + | ===Digestion of oligos with Type II Restriction Enzyme=== | ||
| + | Digesting the oligos with a type II restriction enzyme will result in sticky ends that can anneal to the backbone during the ligation step. | ||
| + | |||
| + | Following the reaction, the oligos should undergo a PCR cleanup. | ||
| + | |||
| + | ===Double digestion of backbone=== | ||
| + | A double digestion on the backbone decreases the likelihood of the backbone self-ligating. When designing the backbone, a Type I cut site was inserted in the cut out gap that resulted from digestion with the Type II restriction enzyme. If the cut out gap is still present on the plasmid (as is the case if the digestion is not completely successful), then the Type I restriction enzyme with cut the plasmid, resulting in blunt ends that are less likely to self-ligate. | ||
| + | |||
| + | Following digestion, the backbone undergoes a phosphatase reaction for an hour, to decrease the likelihood of self-ligation by removing the 5’ phosphate. | ||
| + | |||
| + | Following the phosphatase reaction, the backbone should undergo a PCR cleanup. | ||
| + | |||
| + | ===Ligation of oligo library and backbone=== | ||
| + | When ligating 1000 ng of backbone, and insert:backbone ratio of 2:1 is optimal for our reactions. Prior to adding the ligase, the reaction is heated to 37* for 5 minutes, to prevent any non-specific annealing. Once the reaction cools back down to room temperature, the ligase is added, and the reaction is allowed to run for 10 minutes at room temperature. | ||
| + | |||
| + | The ligation product should be run through a min-elute column, taking care to elute in 10 ul of water. | ||
| + | |||
| + | ===Transformation=== | ||
| + | 100 ng of ligation product should be transformed into library competent cells. | ||
Revision as of 18:10, 23 September 2011
Bioinformatics | Chip Synthesis | Plasmid Construction | Selection Strain Engineering | Selection Results
Chip Synthesis
From Oligo Pool to Living Library
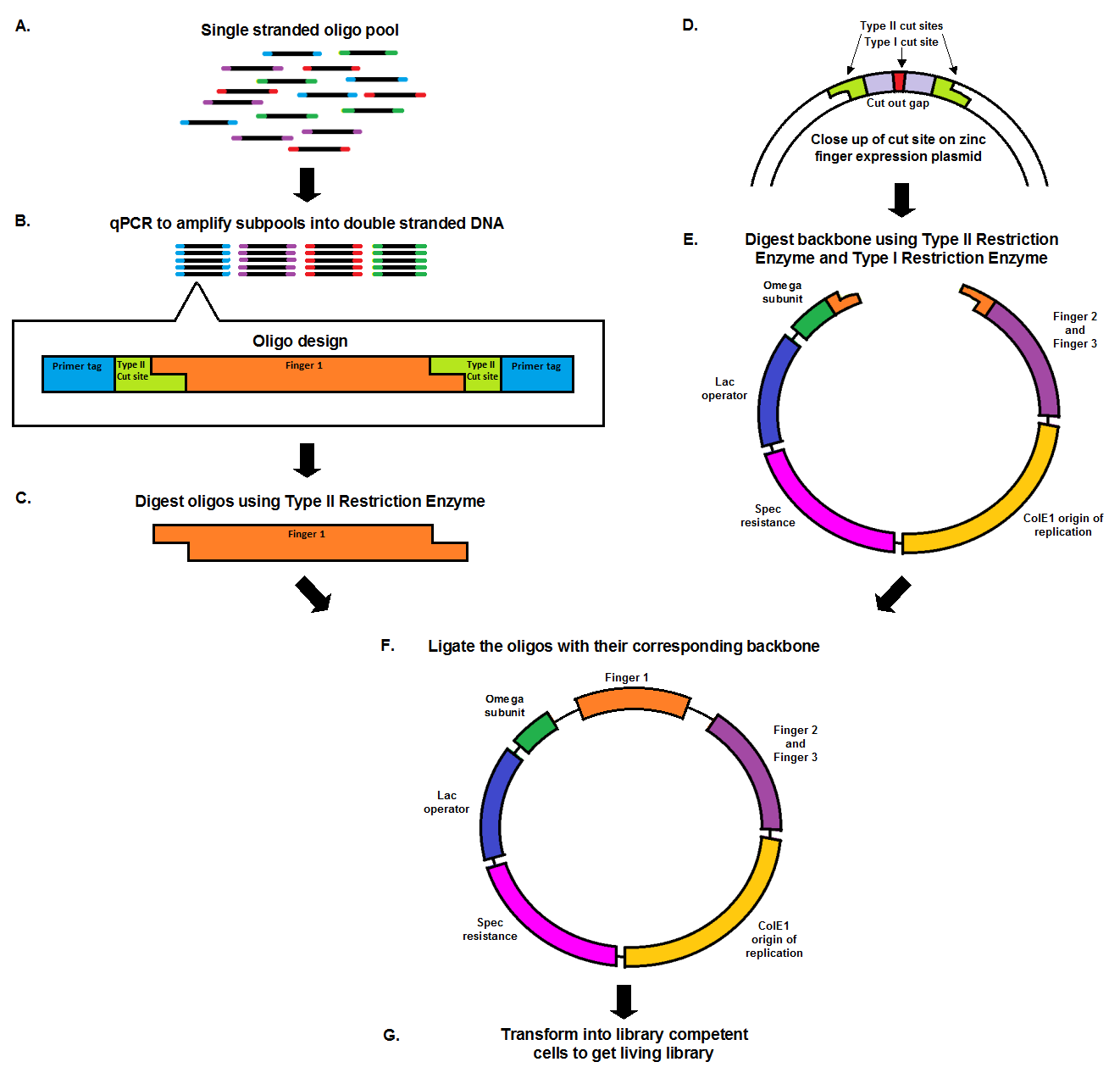
Chip synthesis results in a pool of single stranded oligos (A), designed with primer tags, which allow for the amplification of specific sub-pools, and Type II cut sites. qPCR, which amplifies the oligos at equal rates, results in the desired sub-pool (B). In order to prepare the oligo inserts and expression plasmid backbone for ligation, both must be cut with a Type II restriction enzyme (C, E). The cuts result in complementary sticky ends that anneal to each other during the ligation. The backbone, designed with a second cut site in the cut out gap (D), also undergoes a second digestion with a Type I restriction enzyme in order to minimize the number of undigested, or partially digested backbones. The oligo pool and backbone are then ligated (F), and transformed into library competent cells, resulting in a living library (G).
Protocol
qPCR of subpools
Complete protocol found in the supplementary material of Kosuri 2010
Perform one qPCR reaction for each single stranded sub-pool, using the following recipe:
- 100 ul Sybr Green master mix
- 200 nm primer
- 2 ul pooled oligo
- Water to bring total volume up to 200 ul
When performing the PCR reaction, one wants to watch the graphs produced by the thermocycler. Initially, the reaction will proceed at an exponential rate. However, as soon as it begins to plateau, the reaction should be stopped. Until this point, oligos were being amplified equally. However, once the plateau is reached, oligos are amplified at unqual rates. It will take approximately 10 cycles to reach the plateau.
On the thermocycler, run the following program:
- 1 minute at 95*
- 10 seconds at 95*
- 30 seconds at the primer annealing temperature
- Go to step 2 until the reaction plateaus.
The PCR product should undergo PCR purification.
Digestion of oligos with Type II Restriction Enzyme
Digesting the oligos with a type II restriction enzyme will result in sticky ends that can anneal to the backbone during the ligation step.
Following the reaction, the oligos should undergo a PCR cleanup.
Double digestion of backbone
A double digestion on the backbone decreases the likelihood of the backbone self-ligating. When designing the backbone, a Type I cut site was inserted in the cut out gap that resulted from digestion with the Type II restriction enzyme. If the cut out gap is still present on the plasmid (as is the case if the digestion is not completely successful), then the Type I restriction enzyme with cut the plasmid, resulting in blunt ends that are less likely to self-ligate.
Following digestion, the backbone undergoes a phosphatase reaction for an hour, to decrease the likelihood of self-ligation by removing the 5’ phosphate.
Following the phosphatase reaction, the backbone should undergo a PCR cleanup.
Ligation of oligo library and backbone
When ligating 1000 ng of backbone, and insert:backbone ratio of 2:1 is optimal for our reactions. Prior to adding the ligase, the reaction is heated to 37* for 5 minutes, to prevent any non-specific annealing. Once the reaction cools back down to room temperature, the ligase is added, and the reaction is allowed to run for 10 minutes at room temperature.
The ligation product should be run through a min-elute column, taking care to elute in 10 ul of water.
Transformation
100 ng of ligation product should be transformed into library competent cells.
 "
"