Team:Paris Bettencourt/Experiments/YFP TetR diffusion
From 2011.igem.org
Aleksandra (Talk | contribs) |
|||
| Line 4: | Line 4: | ||
<h1>Experiments of the YFP concentrator design</h1> | <h1>Experiments of the YFP concentrator design</h1> | ||
| - | <p> | + | <p>This system is an improvement of the original experiment with the GFP. In fact, to see the fluorescence better, we decided to concentrate the YFP fluorescent molecules fused to TetR on the TetO array to make them more visible in the cell. We have been kindly given the plasmids containing YFP:TetR construct and TetO array by D. Lane. In order to use this system according to our designs, we biobricked the TetO array and incorporated it in a plasmid compatible with <i>B.subtilis</i> (pHM3). In fact, this plasmid is also compatible with <i>E.coli</i>, which permitted us to successfully characterize it in this strain. The results are presented here in detail.</p> |
<h2>Design overview</h2> | <h2>Design overview</h2> | ||
| Line 26: | Line 26: | ||
<h2>Results</h2> | <h2>Results</h2> | ||
</html> | </html> | ||
| - | Start with the good news : <br>We | + | Start with the good news : <br>We succeeded to biobrick the TetO array and the next step is to characterize it. The plan is to do microscopy in ''E. coli'' double transformed with YFP:tetR WT and tetO array (Biobricked), ''E. coli'' with YFP:tetR WT only, ''E. coli'' with tetO array BB only, <i>B.subtilis</i> with tetO array (in pHM3 or K090403). <br><br> |
The bad news : <br> | The bad news : <br> | ||
| - | We have a lot of trouble to biobrick the YFP:tetR so it will be done if we | + | We have a lot of trouble to biobrick the YFP:tetR so it will be done if we succeed to go to the World Jamboree. |
<html> | <html> | ||
<h3>Testing the YFP:tetR and tetO array strains from D. Lane</h3> | <h3>Testing the YFP:tetR and tetO array strains from D. Lane</h3> | ||
| - | In the article <a href="https://2011.igem.org/Team:Paris_Bettencourt/Experiments/YFP_TetR_diffusion#references">[1]</a>, <i>E. coli</i> strains are growing at 20°C to avoid protein | + | In the article <a href="https://2011.igem.org/Team:Paris_Bettencourt/Experiments/YFP_TetR_diffusion#references">[1]</a>, <i>E. coli</i> strains are growing at 20°C to avoid protein aggregation, but the problem is that nanotubes between <i>B. subtilis</i> have only been shown to exist at 37°C. |
| - | We | + | We tested different possibilities : at 37°C or 30°C and concentration of arabinose (0% - 0,1% -0,2%) to deal with protein aggregation. |
</html> | </html> | ||
<center> | <center> | ||
| Line 54: | Line 54: | ||
<html> | <html> | ||
| - | <p>With the | + | <p>With the combination of YFP:TetR and TetO array plasmids, we can see few spots of fluorescence with 0,2% arabinose and because there are in the cell extremity, we can suppose that it is concentrated fluorescence in TetO array. Nevertheless the protein aggregation is very effective when there is only YFP:tetR at 30°C and 37°C in <i>E. coli</i>.</p> |
<p>More pictures and information on the notebook here : <a href="https://2011.igem.org/Team:Paris_Liliane_Bettencourt/Notebook/2011/08/03/#Kevin">link</a></p> | <p>More pictures and information on the notebook here : <a href="https://2011.igem.org/Team:Paris_Liliane_Bettencourt/Notebook/2011/08/03/#Kevin">link</a></p> | ||
<h3>Characterization: Biobricked TetO Array's running way </h3> | <h3>Characterization: Biobricked TetO Array's running way </h3> | ||
| - | <h4>Microscopy of double | + | <h4>Microscopy of double transformed pFX234 / Biobricked TetO Array <i>E. Coli</i></h4> |
</html> | </html> | ||
| Line 93: | Line 93: | ||
</center> | </center> | ||
| - | The pictures of TetO show no YFP activity, which is normal because there is no YFP sequence in these plasmids.<br> | + | The pictures of TetO alone show no YFP activity, which is normal because there is no YFP sequence in these plasmids.<br> |
| - | The TetR-YFP construct which constitutes the transmitter part, occasionally shows gross aggregated YFP | + | The TetR-YFP construct which constitutes the transmitter part, occasionally shows gross aggregated YFP.<br> |
| - | After observing the | + | After observing the cells carrying both the TetO and YFP:TetR constructs, we can obviously distinguish glowing dots in some cells. They reflect the behavior we expected. Indeed, appearance of dots (red arrow) shows that the receiver (TetO array) actually links tightly to TetR-YFP which is the emitted protein! In fact, you can see a lot of glowing dots, each of them being a concentration of fluorescent molecules (we have highlighted some of them with red arrows). |
<h4>Microscopy of ibpA mCherry double transformated in <i>E. Coli</i></h4> | <h4>Microscopy of ibpA mCherry double transformated in <i>E. Coli</i></h4> | ||
We transformated ibpA mCherry cells (expressing a mcherry in a agregation chaperon protein, with courtesy of Anne-Sophie Coquel, Inserm U1001) with pFX234 YFP:TetR and biobricked TetO Array plasmids to differenciate TetO array foci than agregation. | We transformated ibpA mCherry cells (expressing a mcherry in a agregation chaperon protein, with courtesy of Anne-Sophie Coquel, Inserm U1001) with pFX234 YFP:TetR and biobricked TetO Array plasmids to differenciate TetO array foci than agregation. | ||
| Line 103: | Line 103: | ||
[[File:microscopy_yfp_ibpa.jpg|center|]] | [[File:microscopy_yfp_ibpa.jpg|center|]] | ||
| - | Microscopy shows overlap for most foci and mCherry agregation but there | + | Microscopy shows overlap for most foci and mCherry agregation but there is some foci that is alone.<br> |
Hopefully we don't expect to get high concentration of YFP:tetR in receiver cell so it will be ok. | Hopefully we don't expect to get high concentration of YFP:tetR in receiver cell so it will be ok. | ||
Revision as of 02:34, 22 September 2011

Experiments of the YFP concentrator design
This system is an improvement of the original experiment with the GFP. In fact, to see the fluorescence better, we decided to concentrate the YFP fluorescent molecules fused to TetR on the TetO array to make them more visible in the cell. We have been kindly given the plasmids containing YFP:TetR construct and TetO array by D. Lane. In order to use this system according to our designs, we biobricked the TetO array and incorporated it in a plasmid compatible with B.subtilis (pHM3). In fact, this plasmid is also compatible with E.coli, which permitted us to successfully characterize it in this strain. The results are presented here in detail.
Design overview

Cloning of TetO array construction
More information on the design here : link
Parts and biobrick system construction
YFP:TetR construction

Cloning plan of YFP:TetR construction
TetO array construction

Cloning plan of TetO array construction
Results
Start with the good news :We succeeded to biobrick the TetO array and the next step is to characterize it. The plan is to do microscopy in E. coli double transformed with YFP:tetR WT and tetO array (Biobricked), E. coli with YFP:tetR WT only, E. coli with tetO array BB only, B.subtilis with tetO array (in pHM3 or K090403).
The bad news :
We have a lot of trouble to biobrick the YFP:tetR so it will be done if we succeed to go to the World Jamboree.
Testing the YFP:tetR and tetO array strains from D. Lane
In the article [1], E. coli strains are growing at 20°C to avoid protein aggregation, but the problem is that nanotubes between B. subtilis have only been shown to exist at 37°C. We tested different possibilities : at 37°C or 30°C and concentration of arabinose (0% - 0,1% -0,2%) to deal with protein aggregation.
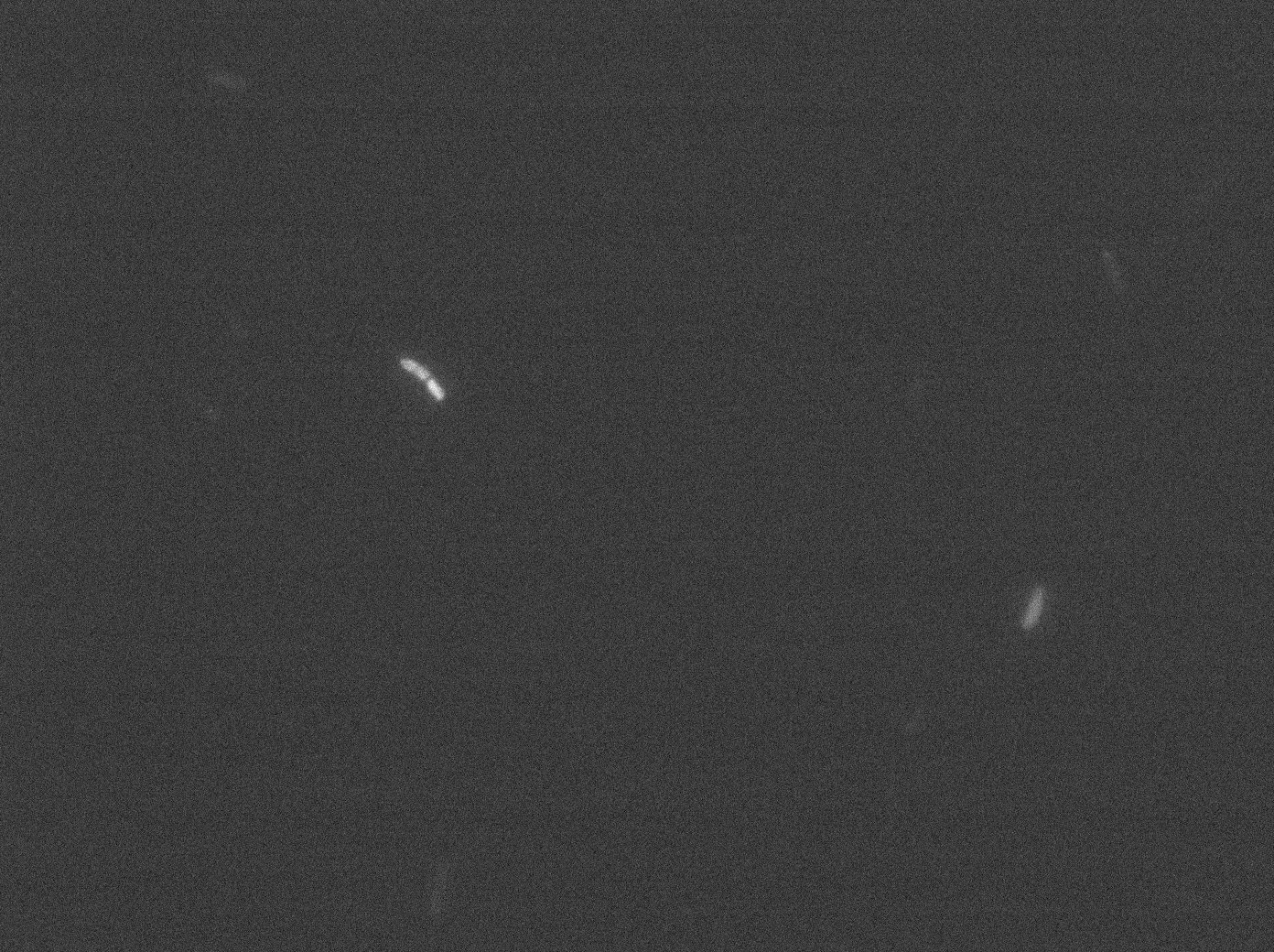
With the combination of YFP:TetR and TetO array plasmids, we can see few spots of fluorescence with 0,2% arabinose and because there are in the cell extremity, we can suppose that it is concentrated fluorescence in TetO array. Nevertheless the protein aggregation is very effective when there is only YFP:tetR at 30°C and 37°C in E. coli.
More pictures and information on the notebook here : link
Characterization: Biobricked TetO Array's running way
Microscopy of double transformed pFX234 / Biobricked TetO Array E. Coli
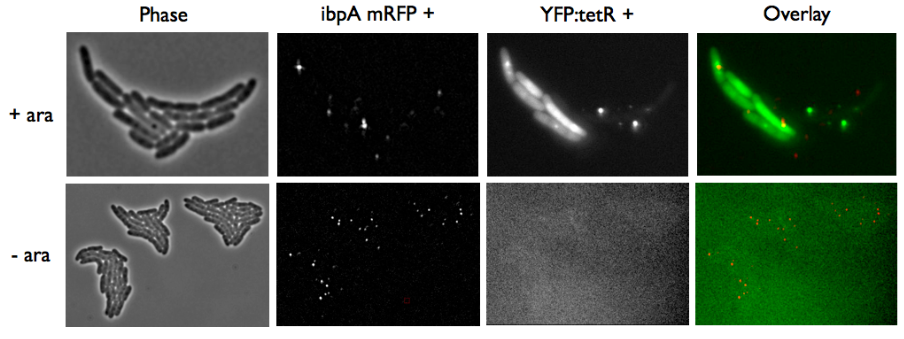
In order to do this characterization, we took pictures of different plasmids containing only TetO; TetR + YFP; TetO + TetR + YFP. in each case we made a control by non inducing the promoter with arabinose in E. coli (double transformated with pFX234 and TetO Array).
The pictures of TetO alone show no YFP activity, which is normal because there is no YFP sequence in these plasmids.
The TetR-YFP construct which constitutes the transmitter part, occasionally shows gross aggregated YFP.
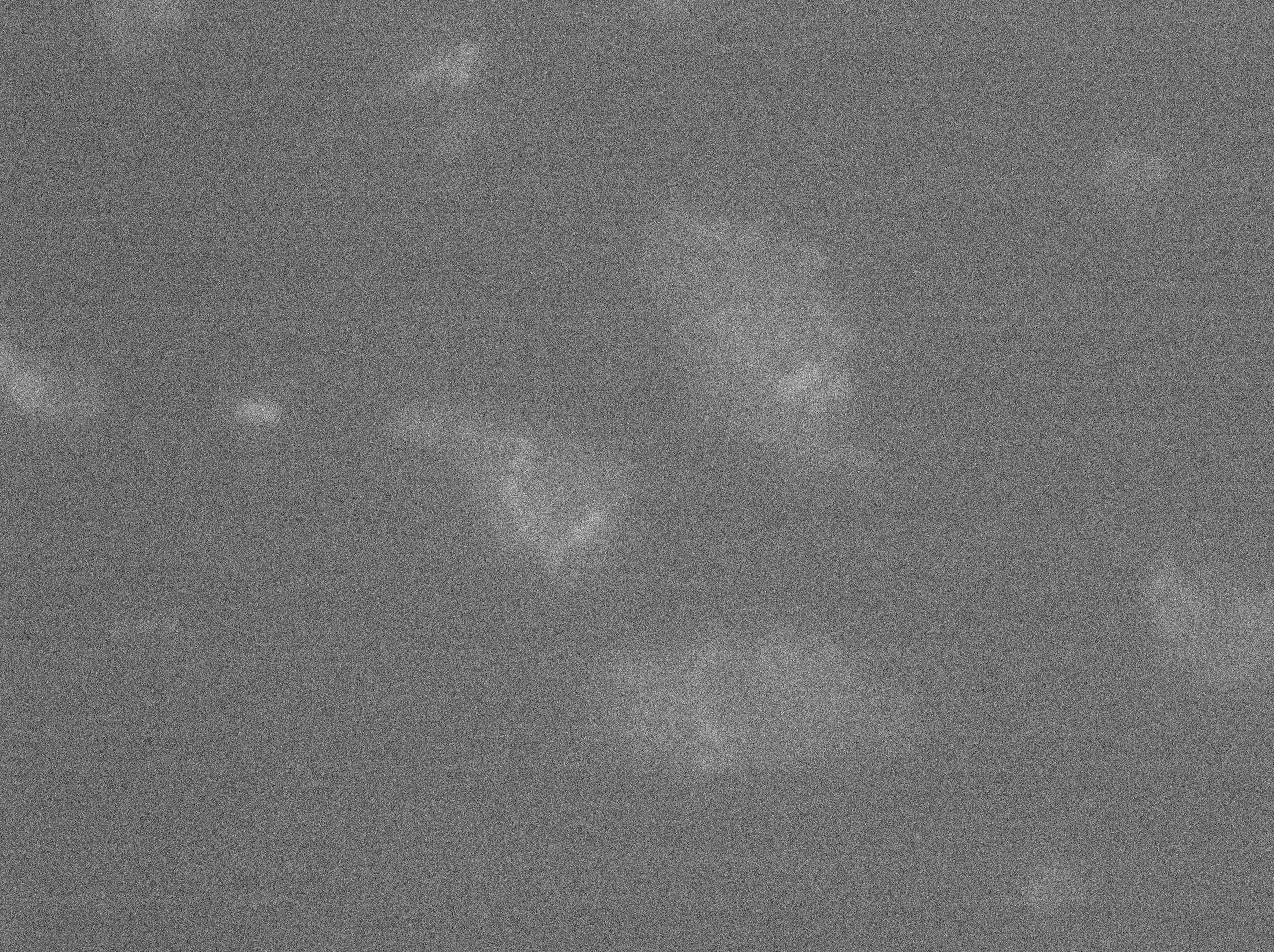
After observing the cells carrying both the TetO and YFP:TetR constructs, we can obviously distinguish glowing dots in some cells. They reflect the behavior we expected. Indeed, appearance of dots (red arrow) shows that the receiver (TetO array) actually links tightly to TetR-YFP which is the emitted protein! In fact, you can see a lot of glowing dots, each of them being a concentration of fluorescent molecules (we have highlighted some of them with red arrows).
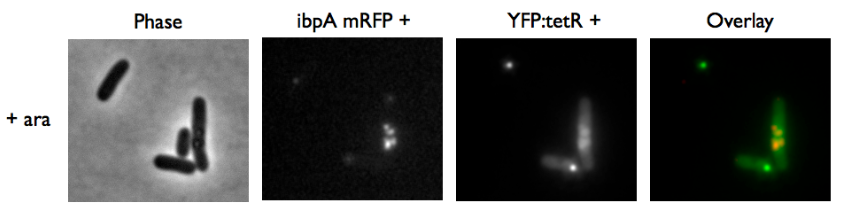
Microscopy of ibpA mCherry double transformated in E. Coli
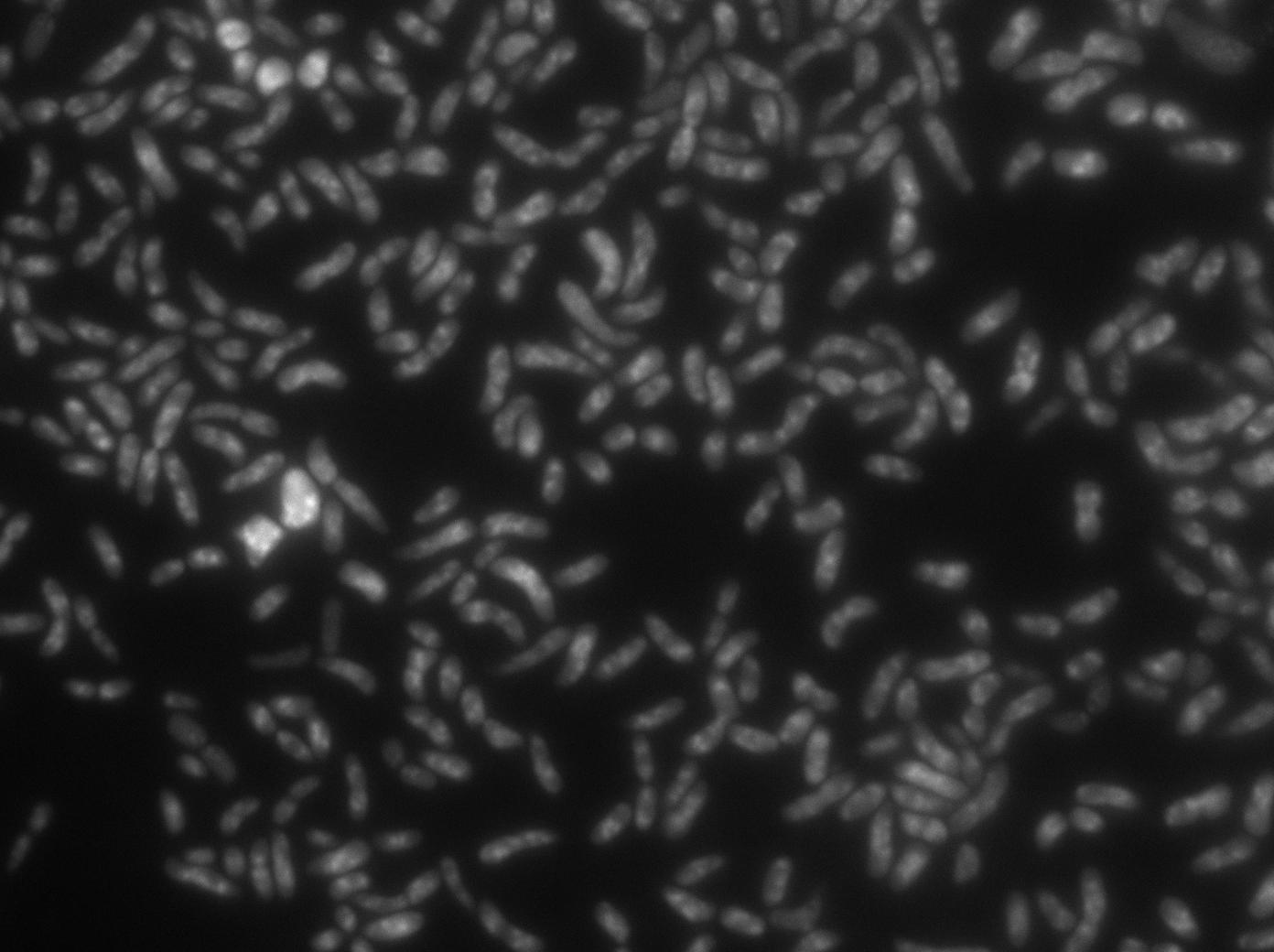
We transformated ibpA mCherry cells (expressing a mcherry in a agregation chaperon protein, with courtesy of Anne-Sophie Coquel, Inserm U1001) with pFX234 YFP:TetR and biobricked TetO Array plasmids to differenciate TetO array foci than agregation.
- Case of YFP:TetR over-expression by arabinose induction
Microscopy shows overlap for most foci and mCherry agregation but there is some foci that is alone.
Hopefully we don't expect to get high concentration of YFP:tetR in receiver cell so it will be ok.
- Case of YFP:TetR low expression by arabinose induction
Microscopy shows that most of agregation are gone and we have more not-overlaping foci.
We could manage to get less agregation if we deal with the arabinose induction.
Future
Next step is to biobrick the YFP:TetR fusion protein so we can finish the cloning plan and put the system in B. subtilis. Hopefully we can improve our GFP diffusion experiments and have a better characterisation of nanotubes !References and acknowledgments
- Kinetics of plasmid segregation in Escherichia coli, Scott Gordon, Jerôme Rech, David Lane and Andrew Wright, Molecular Biology, available here Thanks to David Lane, Andrew Wright and François-Xavier Barre for information and great help they gave to us
 "
"