Team:UCL London/Research/Stresslights/Experiments
From 2011.igem.org
| Line 72: | Line 72: | ||
</tr> | </tr> | ||
</table></div></html> | </table></div></html> | ||
| + | |||
| + | <h2>Procedure for preparing BioBrick</h2> | ||
| + | # Carried out digestion of the mNARK promoter and YFP.LVA protein on their relevant plasmid backbones with EcoRI and SpeI. Also digested RBS and Terminator on their respective plasmid backbones with XbaI and PstI | ||
| + | # Carried out digestion of linearised pSB1K3, pSB1T3, pSB1C3 plasmid backbones with EcoRI and PstI | ||
| + | # Performed gel electrophoresis with a sample from all the digestion mixtures to confirm the parts | ||
| + | # Carried out ligation of the mNARK, RBS and pSB1K3 plasmid. Also ligated YFP.LVA, terminator and pSB1T3. | ||
| + | # Transformed separate stocks of competent TOP10 E. coli cells with the ligation mixtures and grew them overnight on antibiotic plates and then selective LB medium. | ||
| + | # Mini-prepped the overnight cultures to extract the plasmids. | ||
| + | # Digested the mNark/RBS/pSB1K3 with EcoRI and SpeI. Also digested YFP.LVA/Terminator/pSB1T3 with XbaI and PstI | ||
| + | # Performed gel electrophoresis with a sample from both digestion mixtures to confirm the composite parts | ||
| + | # Carried out ligation of mNark/RBS, YFP.LVA.TER and pSB1C3 | ||
| + | # Transformed a stock of competent TOP10 E. coli cells with the ligation mixture and grew the cells overnight on chloramphenicol plates, followed by overnight culture in selective LB medium | ||
| + | # Set up a glycerol stock from overnight culture (for storage at -80 °C) and mini-prepped the rest to extract plasmid with the final construct into 50 μl sample | ||
| + | # Carried out digestion of mini-prep sample with EcoRI and PstI and performed gel electrophoresis to confirm the length of the final construct on gel | ||
| + | # Sent a sample of the mini-prep for sequencing to confirm the final construct | ||
| + | # Submitted the improved BioBrick (BBa_K676002) to the registry | ||
| + | |||
| + | <h2> Parts submitted to Registry</h2> | ||
| + | <html><div align="center"><table border="1" cellspacing="0" cellpadding="2"> | ||
| + | <tr> | ||
| + | <td width="180" valign="top" bgcolor="#CCCCCC"><p><strong>Part Name</strong></p></td> | ||
| + | <td width="91" valign="top" bgcolor="#CCCCCC"><p align="center"><strong>Parts ID</strong></p></td> | ||
| + | <td width="151" valign="top" bgcolor="#CCCCCC"><p align="center"><strong>Parts Description</strong></p></td> | ||
| + | <td width="100" valign="top" bgcolor="#CCCCCC"><p align="center"><strong>Length (bp)</strong></p></td> | ||
| + | <td width="200" valign="top" bgcolor="#CCCCCC"><p align="center"><strong>Plasmid Backbone</strong></p></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td width="77" valign="top"><p>mNARK with YFP.LVA</p></td> | ||
| + | <td width="91" valign="top"><p><a href="http://partsregistry.org/wiki/index.php?title=Part:BBa_K676002">BBa_K676002</a></p></td> | ||
| + | <td width="151" valign="top"><p>Improved oximeter for detecting hypoxia and express YFP</p></td> | ||
| + | <td width="53" valign="top"><p align="center">1014</p></td> | ||
| + | <td width="109" valign="top"><p>on pSB1C3 (2070 bp) - chloramphenicol resistance</p></td> | ||
| + | </tr> | ||
| + | </table></div></html> | ||
| + | |||
</div> | </div> | ||
{{:Team:UCL_London/Template/Footer}} | {{:Team:UCL_London/Template/Footer}} | ||
Revision as of 02:03, 22 September 2011
Contents |
Modifying Oximeter in the lab
Aim
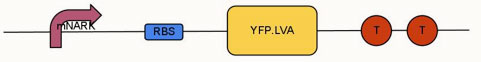
To modify and improve the oximeter device (BBa_K239011) using modified nark promoter (BBa_K239006) submitted by 2009 UCL iGEM team. Changes included replacing the normal GFP (green fluorescent protein) with an enhanced YFP (yellow fluorescent protein), which has an LVA tag added to its C terminal.
Parts used
Part Name |
Parts ID |
Parts Description |
Length (bp) |
Plasmid Backbone |
mNARK promoter |
promoter sensitive to hypoxia |
89 |
on pUC57 (2710 bp) - ampicillin resistance |
|
RBS |
ribosome binding site |
12 |
on pSB1A2 (2079 bp) - ampicillin resistance |
|
YFP.LVA |
enhanced yellow fluorescent protein with LVA tag |
759 |
on pSB1A2 (2079 bp) |
|
Terminator |
double terminator (B0010-B0012) |
129 |
on pSB1A2 (2079 bp) |
|
n/a |
standard plasmid backbone (kanamycin resistance) |
2204 |
n/a |
|
n/a |
standard plasmid backbone (tetracycline resistance) |
2463 |
n/a |
|
n/a |
standard plasmid backbone (chloramphenicol resistance) |
2070 |
n/a |
Procedure for preparing BioBrick
- Carried out digestion of the mNARK promoter and YFP.LVA protein on their relevant plasmid backbones with EcoRI and SpeI. Also digested RBS and Terminator on their respective plasmid backbones with XbaI and PstI
- Carried out digestion of linearised pSB1K3, pSB1T3, pSB1C3 plasmid backbones with EcoRI and PstI
- Performed gel electrophoresis with a sample from all the digestion mixtures to confirm the parts
- Carried out ligation of the mNARK, RBS and pSB1K3 plasmid. Also ligated YFP.LVA, terminator and pSB1T3.
- Transformed separate stocks of competent TOP10 E. coli cells with the ligation mixtures and grew them overnight on antibiotic plates and then selective LB medium.
- Mini-prepped the overnight cultures to extract the plasmids.
- Digested the mNark/RBS/pSB1K3 with EcoRI and SpeI. Also digested YFP.LVA/Terminator/pSB1T3 with XbaI and PstI
- Performed gel electrophoresis with a sample from both digestion mixtures to confirm the composite parts
- Carried out ligation of mNark/RBS, YFP.LVA.TER and pSB1C3
- Transformed a stock of competent TOP10 E. coli cells with the ligation mixture and grew the cells overnight on chloramphenicol plates, followed by overnight culture in selective LB medium
- Set up a glycerol stock from overnight culture (for storage at -80 °C) and mini-prepped the rest to extract plasmid with the final construct into 50 μl sample
- Carried out digestion of mini-prep sample with EcoRI and PstI and performed gel electrophoresis to confirm the length of the final construct on gel
- Sent a sample of the mini-prep for sequencing to confirm the final construct
- Submitted the improved BioBrick (BBa_K676002) to the registry
Parts submitted to Registry
Part Name |
Parts ID |
Parts Description |
Length (bp) |
Plasmid Backbone |
mNARK with YFP.LVA |
Improved oximeter for detecting hypoxia and express YFP |
1014 |
on pSB1C3 (2070 bp) - chloramphenicol resistance |
 "
"