Team:Paris Bettencourt/Experiments/T7 diffusion
From 2011.igem.org
Danyel.lee90 (Talk | contribs) |
|||
| Line 34: | Line 34: | ||
<h3>Growth</h3> | <h3>Growth</h3> | ||
| - | <p>The measurements have been carried out on a spectrophotometer, at 37°C under | + | <p>The measurements have been carried out on a spectrophotometer, at 37°C under transient shaking, for 4h, for several colonies and several range of IPTG concentration. The OD 600nm and the fluorescence of the GFP (exc: 470nm / meas:515 nm) was measured every 5 min, and the ratio of the two was calculated.</p> |
<p>All values were normalized by subtracting the fluorenscence/OD value of the well with 0 mM IPTG at time 0. The values given are in arbitrary units.</p> | <p>All values were normalized by subtracting the fluorenscence/OD value of the well with 0 mM IPTG at time 0. The values given are in arbitrary units.</p> | ||
Revision as of 23:00, 21 September 2011

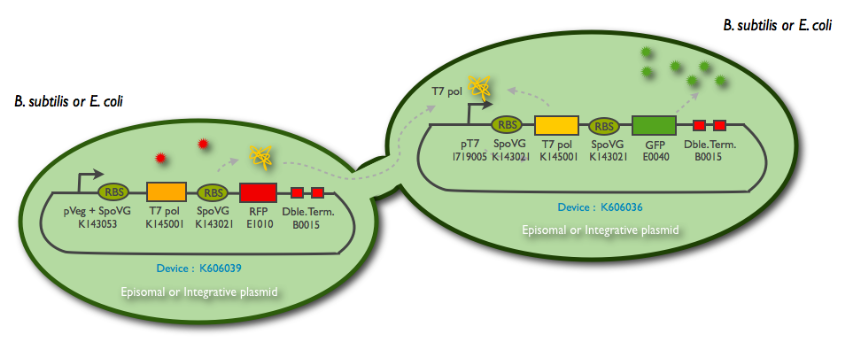
T7 diffusion experiments
Design overview
 Fig1: Schematic summary of the T7 diffusion device
Fig1: Schematic summary of the T7 diffusion device
More information on the design here : link
Parts
The cloning for this system, that seems simple at the beginning took us a very long time, because of many issues we encountered along the cloning.
The first row of problems come from wrong parts sent by the registry. We lost almost one month and an half in building the system (see the corresponding page). We synthetized ourself the pHyperSpank promoter (K143055), but we didn't have the time to incorporate it into our biggest construction. Instead, we cloned a pVeg SpoVG promoter (K143053) that have a constitutive expression.
The following schematic represents what we have indeed managed to clone:
This summary shows the parts we have characterized, and the ones we have sent to the registry. But we have unsuccessfully managed to clone them into an integration (K090403) and a replicative (pHM3) vector for subtilis, as well as the combination of the emittor and the receiver inside the same plasmid.
Characterization of the T7 promoter
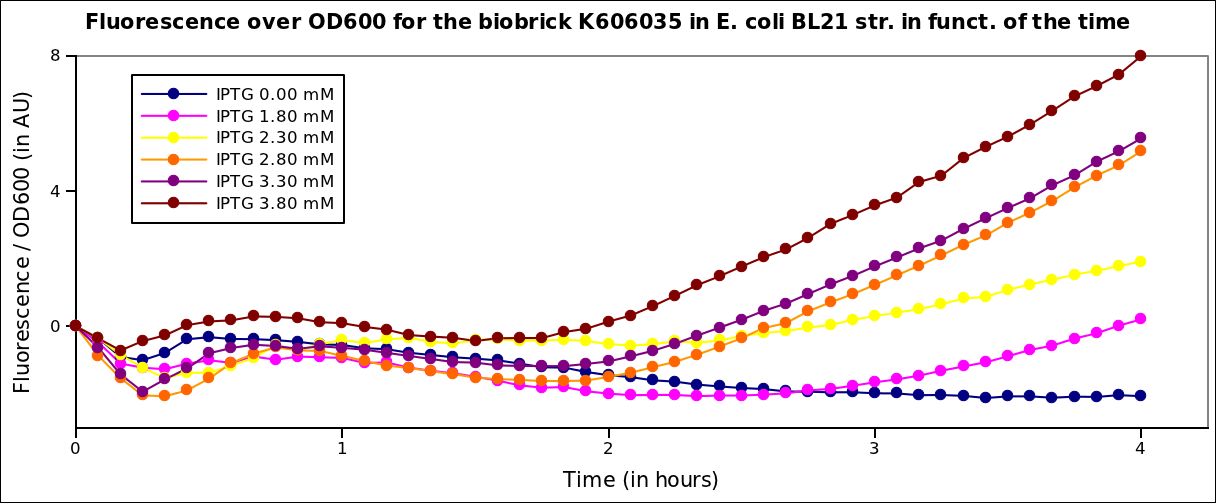
Growth
The measurements have been carried out on a spectrophotometer, at 37°C under transient shaking, for 4h, for several colonies and several range of IPTG concentration. The OD 600nm and the fluorescence of the GFP (exc: 470nm / meas:515 nm) was measured every 5 min, and the ratio of the two was calculated.
All values were normalized by subtracting the fluorenscence/OD value of the well with 0 mM IPTG at time 0. The values given are in arbitrary units.
First, we see an inflexion in the curve that is due to the stong influence of the IPTG on the metabolism of the cells. Then, the bacteria start growing again. We see a clear increase of the fluorescence with the IPTG concentration, that is to say with the quantity of T7 polymerase in the cell.
Here, we plot the ratio of induction of the T7 polymerase dependant construct for the different concentrations of IPTG at a given time (4 hrs), taking the well with 0 IPTG at time 0 as the reference.
Characterization of the T7 signal amplification leakage in E. coli
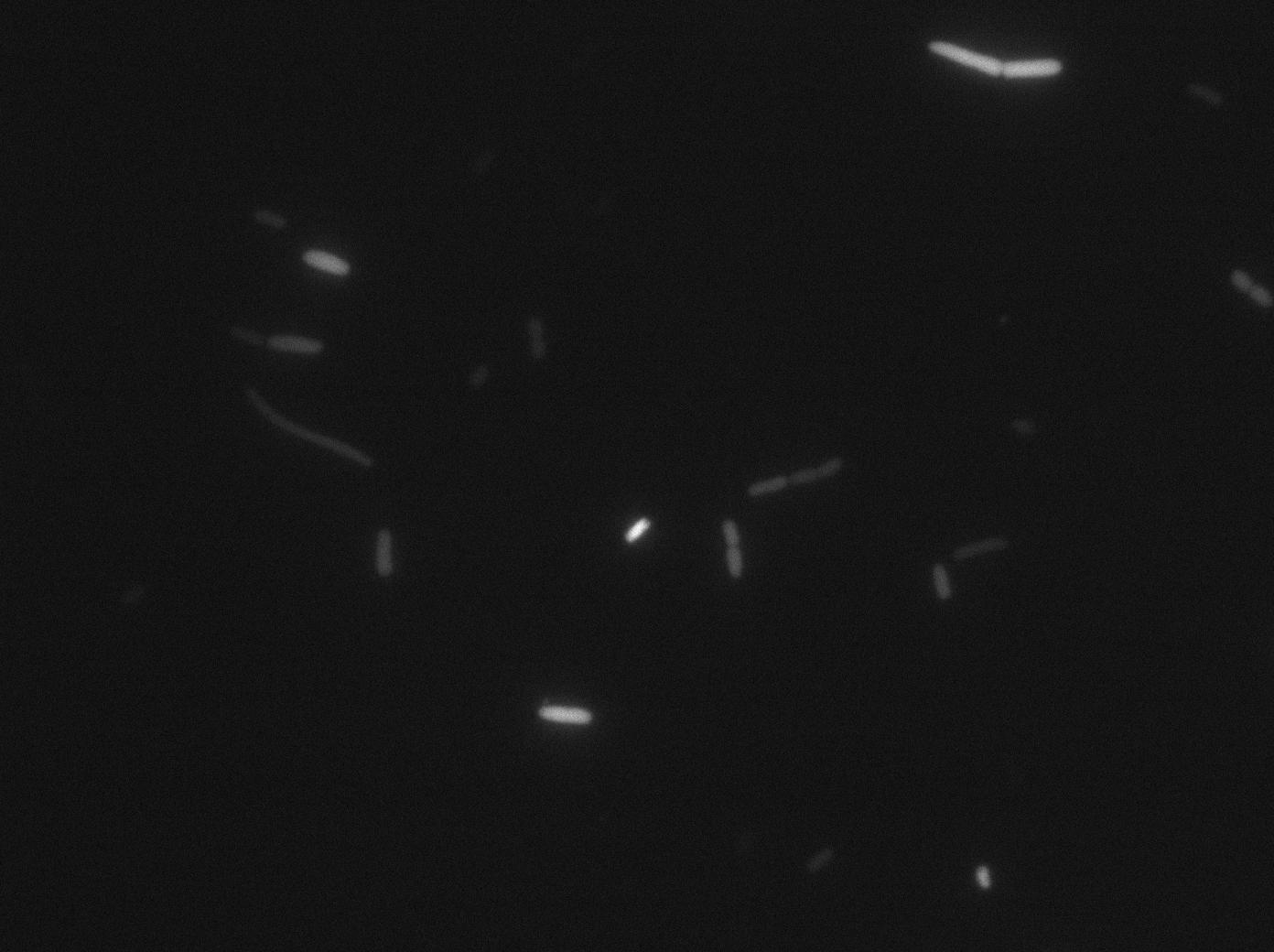
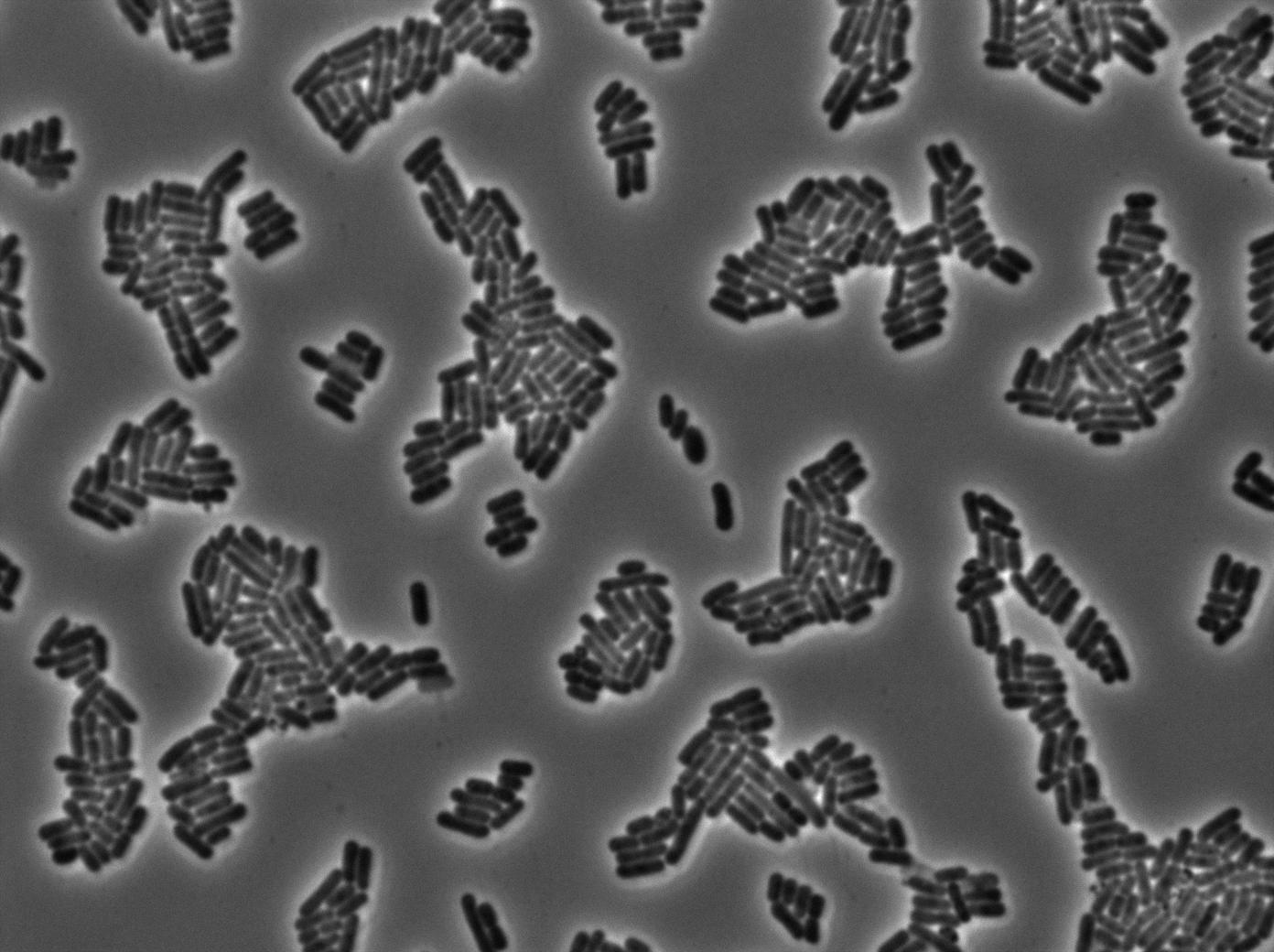
We characterized T7 autoloop in E.coli, when hosted in the plasmid pSB1C3 (K606036). The idea was to see if the system was leaky inside a synthetic biology plasmid, that is to say, a plasmid holding 4 terminators before any construct.
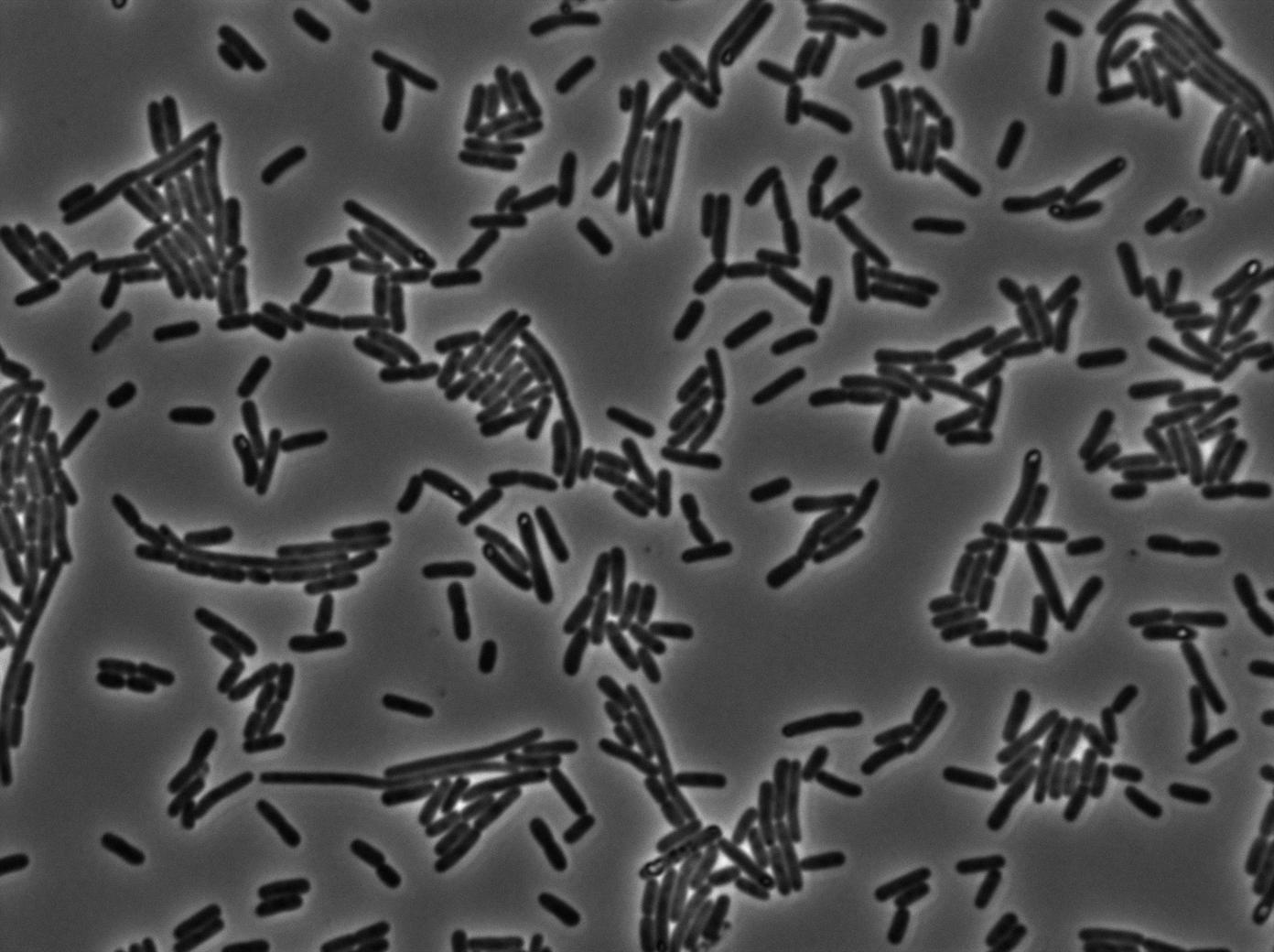
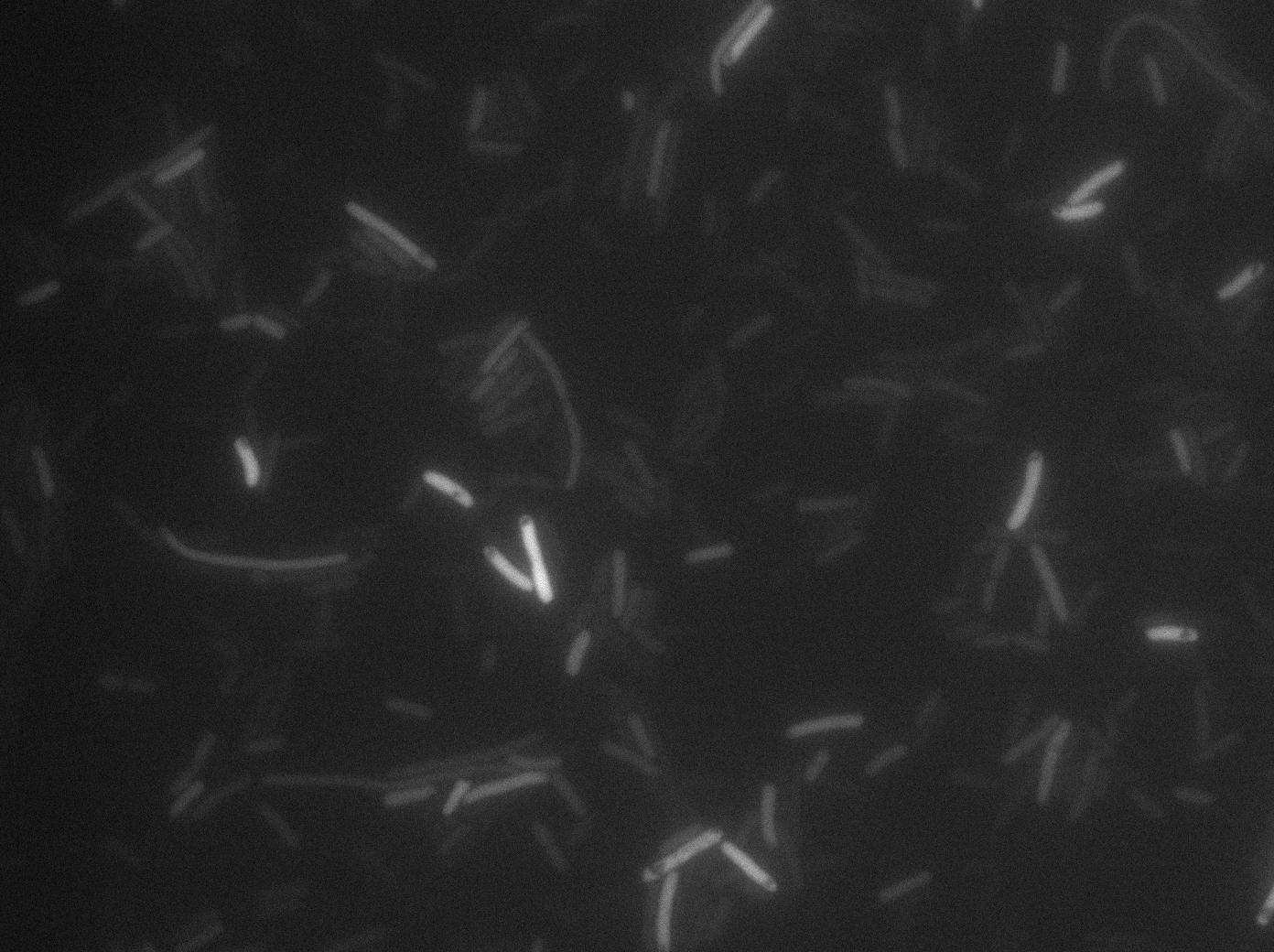
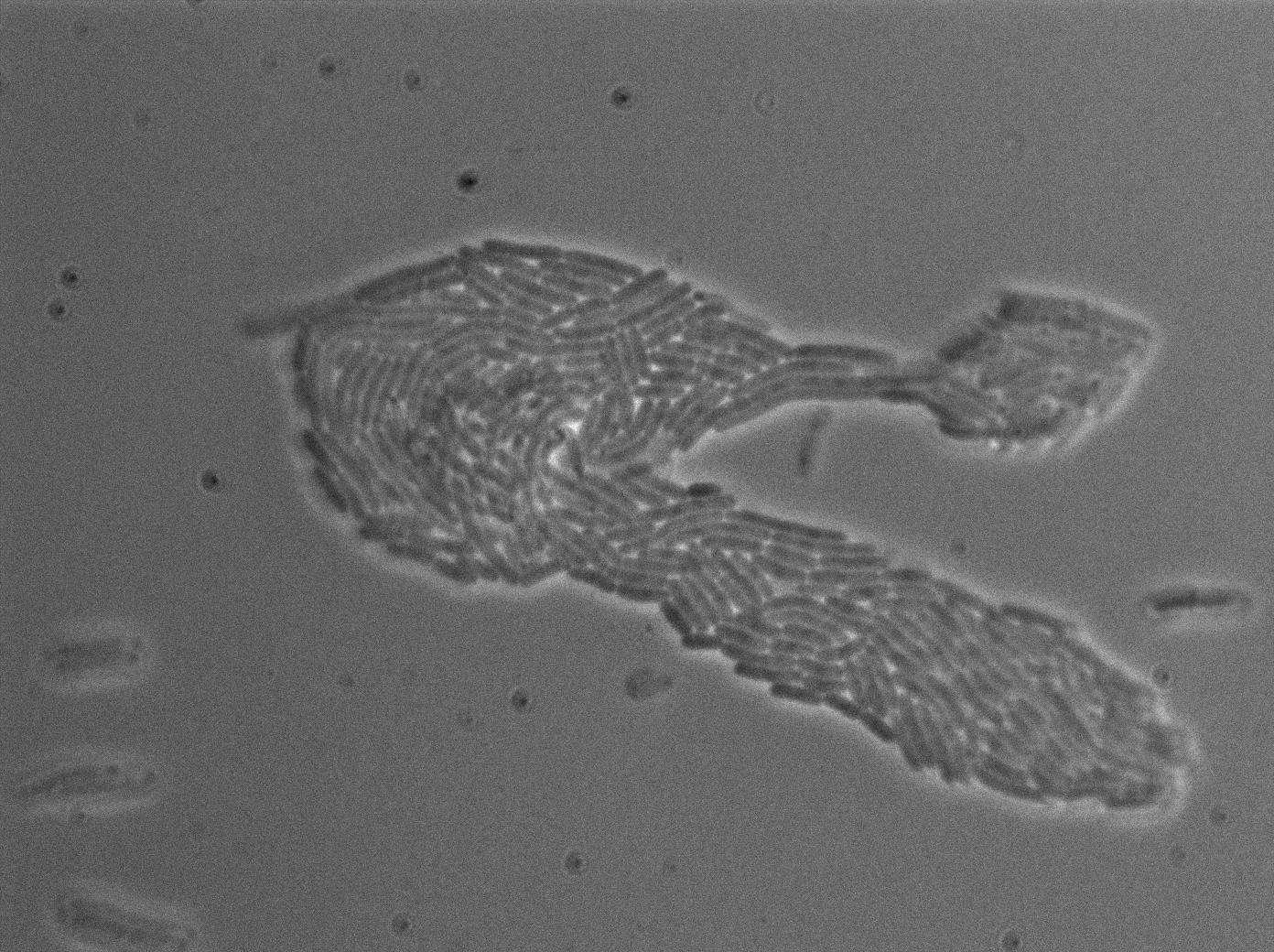
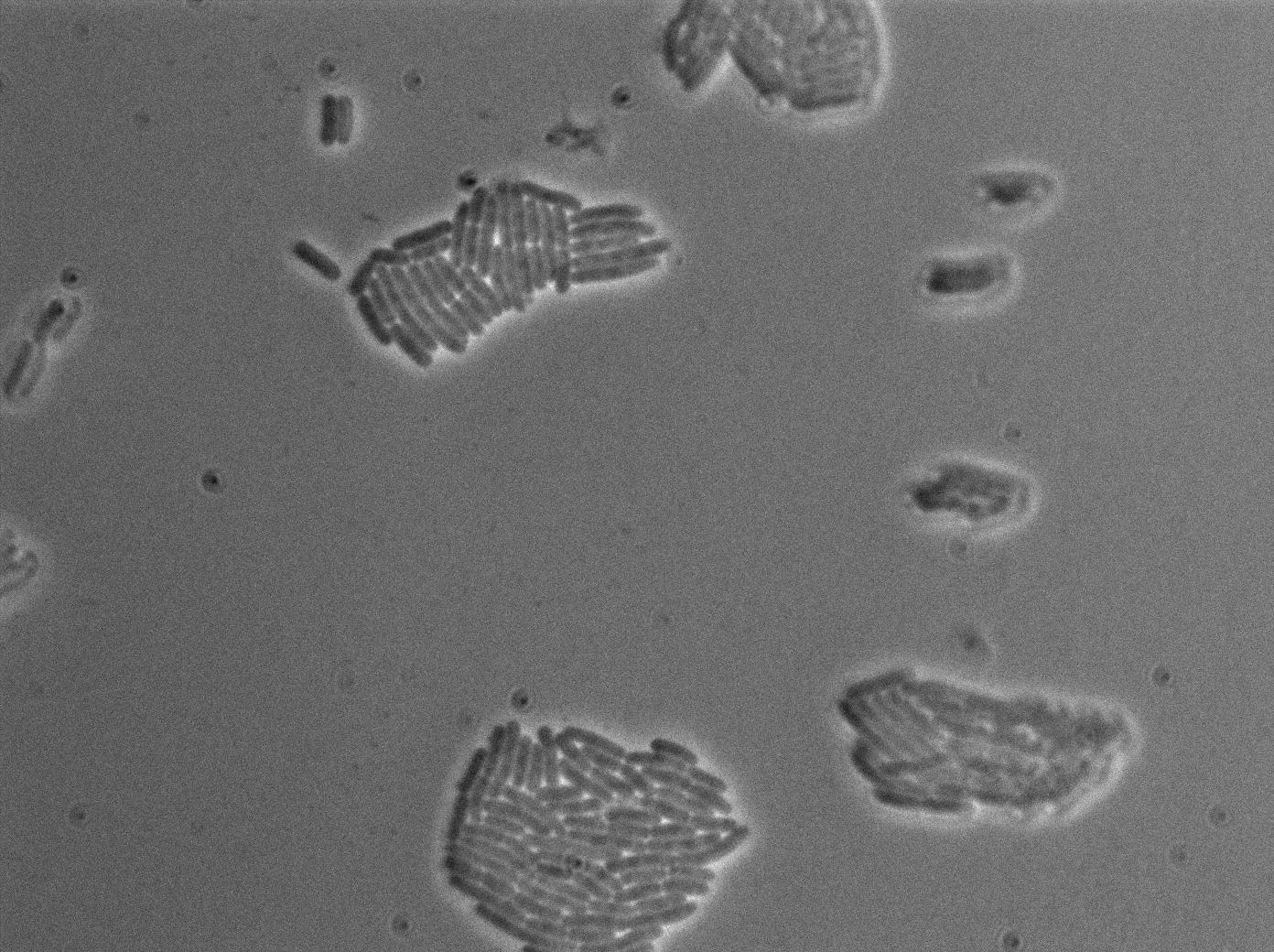
We found out that there is a leakage, but it is small. The cells in which the positive feedback loop is activated stop dividing and glow with a very strong signal. Here are the pictures commented:
The first pictures show that RFP construct has been well done. Indeed, we can see that some cells are glowing with RFP fluorescence. This also shows that the system is not as leaky as we expected for. Indeed, the promoter regulating RFP expression is 'Pveg' which is a constitutive promoter.
Finallyr, the autoloop system is working very well because when leak occurs, cells glow highly.
These pictures show that GFP system gives more disparity than the RFP construct. This is probably due to the LB that already glows in green. It is also noticeable that B.subtilis is naturally shining green. Moreover, the absence of double terminator could explain the leakiness of our system. However, we expected this cells to glow in RFP but they did not. This shows that the full construct has failed.
The last pictures show less disparity that the previous one. This shows that double terminator actually proceeds on the phenotype. We also can notice that the cells glow in red a bit. However, we can not say if it is an artifact or if the system is actually well constructed with the terminator and RFP construct.
Future
At the beginning, we want to clone the LacI expression system (K606054) into the emitter cell (K606039) in order to get the T7 controlled by LacI and thus to improve the expression of T7 polymerase.
Afterwards, we plan to insert these BioBricks in a replicative vector like pHM3 or an integrative vector (like K090403) for B.subtilis in order to test the communication and the transfer of T7 polymerase through the nanotubes and so to characterize them.
 "
"