Team:Paris Bettencourt/Designs
From 2011.igem.org
| Line 19: | Line 19: | ||
<p>The nanotube discorevy is very recent. Though, lots of question remains unsolved, on the processes of <em>the formation of the tubes</em>, the efficiency of the communication, the extent of the process. The existence itself remains contested by some scientists.</p> | <p>The nanotube discorevy is very recent. Though, lots of question remains unsolved, on the processes of <em>the formation of the tubes</em>, the efficiency of the communication, the extent of the process. The existence itself remains contested by some scientists.</p> | ||
| - | <p>We aim to add additionnal proof of their existence and increase the number of the nanotubes, and characterize better the extent of the phenomenon. Here is the list of the question we are asking ourself, and that our designs aimed to | + | <p>We aim to add additionnal proof of their existence and increase the number of the nanotubes, and characterize better the extent of the phenomenon. Here is the list of the question we are asking ourself, and that our designs aimed to answer:<p> |
<table> | <table> | ||
| Line 30: | Line 30: | ||
<li>What is the <em>speed</em> of the process? (passive or active transport)</li> | <li>What is the <em>speed</em> of the process? (passive or active transport)</li> | ||
<li>Can we pass all kind of molecules?</li> | <li>Can we pass all kind of molecules?</li> | ||
| - | <li> | + | <li>Does the communication happen only in between B. subtilis, or can it happen also interspecies or even between E. coli strains?</li> |
<li>If a communication is established with a Gram positive bacteria, is the communication happening through the periplasm of the cytoplasm?</li> | <li>If a communication is established with a Gram positive bacteria, is the communication happening through the periplasm of the cytoplasm?</li> | ||
</ul> | </ul> | ||
| Line 41: | Line 41: | ||
| - | <p>We started designing the project with these | + | <p>We started designing the project with these previously stated questions in questions in mind. Using several molecules of <em>different sizes</em>, from a T7 polymerase to a tRNA amber supressor, we aim to test the capability and size limit for the molecules that can pass through the tubes. But, if the diffusion is passive, the speed of diffusion should be proportional to the diffusion coefficient D, that is to say inversely proportional to the radius of the object. Can we design a system fast-responding enough that would allow us to measure this speed of the transfert?</p> |
</p>We decided that our constructs should follow the global idea summed up in the following picture:</p> | </p>We decided that our constructs should follow the global idea summed up in the following picture:</p> | ||
Revision as of 22:08, 21 September 2011

Designs overview
Introduction
|
Our goal for this summer is to characterize the exchange of proteins through nanotubes as best as we can. In order to do that, we want to construct several designs. In this page, we are going to explain you the principle of the different designs we have built. In the original paper, the authors observe directly the molecules that pass through the nanotubes. The idea behind our approach is to use synthetic biology methods to go farther on the sensitivity and the resolution of these experiments. Relying on signal amplification methods and natural and artificial bistable switches to detect at the molecule resolution when a few molecules have diffused from one emittor cell to the receiver. |
 |
Main questions behind
The nanotube discorevy is very recent. Though, lots of question remains unsolved, on the processes of the formation of the tubes, the efficiency of the communication, the extent of the process. The existence itself remains contested by some scientists.
We aim to add additionnal proof of their existence and increase the number of the nanotubes, and characterize better the extent of the phenomenon. Here is the list of the question we are asking ourself, and that our designs aimed to answer:

|
|
Specification of our designs
We started designing the project with these previously stated questions in questions in mind. Using several molecules of different sizes, from a T7 polymerase to a tRNA amber supressor, we aim to test the capability and size limit for the molecules that can pass through the tubes. But, if the diffusion is passive, the speed of diffusion should be proportional to the diffusion coefficient D, that is to say inversely proportional to the radius of the object. Can we design a system fast-responding enough that would allow us to measure this speed of the transfert?
We decided that our constructs should follow the global idea summed up in the following picture: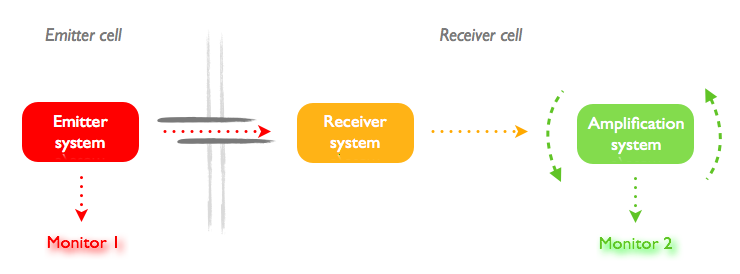
The problem is that each design needs a different couple of signal emitter/receptor. However, even with good models, the response time cannot be predicted easily because little is known about the transfer through the nanotubes. We tried to limit the impact of this issue by the following measures:
- Use each time the same actuator and the same monitor to have comparable response times in the different systems.
- The relevant measure is not the response time itself, but the increase of response time when the two constructs are in the same cell or in different cells linked by a nanotube
The idea is if we can measure this increase of time for different sized, that is to say of different D coefficien, of the molecule we should get a relation in lambda/D, the diffusion is an active process. Else, the process is active. See the modeling pages for more details about this assuption.
In B.subtilis, nanotubes are reported to be formed by the cytoplasm. We had to find a molecule that can be expressed in the first cell that can trigger a genetic circuit in the second cell. The image below illustrates the idea:
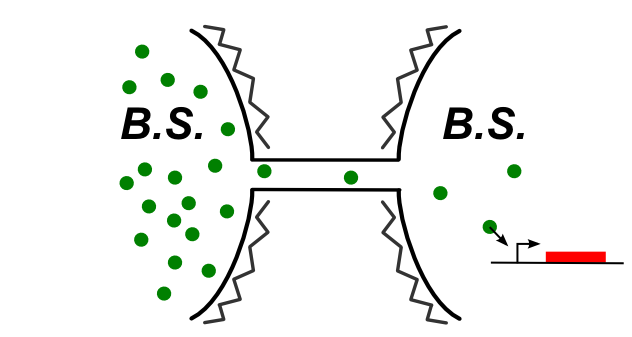
Building fast new genetic devices
Using these specification, we designed several sensitive genetic circuit that can be trigerred by molecules of various size. The molecules chosen cover 2 orders of magnitude of Radius. Here, they are classified from the biggest to the smallest.
| Description | Type of switch | B. subilis E. coli compatibility |
|
|---|---|---|---|

|
T7 polymerase diffusion: The T7 RNA polymerase is the RNA polymerase of the T7 phage. This is a big molecule, that recognizes a very specific promoter orthogonal to B.subtilis. | Irreversible amplification |
Yes |

|
Amber suppressor tRNA diffusion: The principle of this design is to produce in one cell an amber supressor tRNA that will diffuse through the nanotubes. The receptor cell holds the gene for T7 with amber stop codons that cannot be translated into a functional protein as long as the tRNA amber suppressor is not present in the cell. Once expressed, the functional amber T7 RNA polymerase will trigger the T7 amplification system. | Irreversible amplification |
Yes |

|
KinA diffusion: The Sin operon system controls the sporulation switch of B. subtilis. Making it diffuse from a non sporulating strain to a receiver cell makes the receiver cell to sporulate. | Switch | Mono- directional |
Lambda switch
|
Lambda switch design: The CI is a regulation protein from the lambda phage switch. Make it diffuse would change the state of the toogle switch from the push-on push-off system, from the PKU 2007. | Switch | Mono- directional |

|
The YFP-TetR/TetO array experiment: This experiment is an improvement of the previous one. To observe significant fluorescence in the neighboring cell, lots of molecules have to pass through the tubes. Using the affinity of the TetR for the TetO array, we want to concentrate at one point the YFP molecules to better observe this diffusion. | - | Yes |

|
ComS diffusion: ComS is an inhibitor of the MecA protease in B.subtilis. It plays a key role in the triggering of the competence and sporulation mechanisms. The idea is to trigger the switch of the MeKS system of the receptor cell by diffusing ComS through the nanotubes. A major precaution is to prevent the first cell from sporulating. | Switch | Mono- directional |
Others designs we had no time to build
We neither had time nor the manpower to try all the ideas we had. In particular, we have designed some systems in order to investigate the E. coli / B. subtilis connection nature. He had no time to investigate this question further. Nonetheless, we present some of our design ideas for solving this problem in this section.
B.subtilis is Gram- so the nanotubes seem to create a link from the cytoplasm of the first cell to the cytoplasm of the second cell. In the case of the Gram+ bacteria, like E.coli, we wonder which membrane forms the nanotubes . Do they create a link with the periplasm, or with the cytoplasm? To test the two hypotheses, we tried to create a design for each of the two possibilities. If one work and the other does not, the answer to this question will be known.
Design for B.subtilis / E. coli if the connection happens with the cytoplasm
If the connection between E. coli and B. subtilis is happening through the cytoplasm, the type of connection could be summed up by the following schematic.
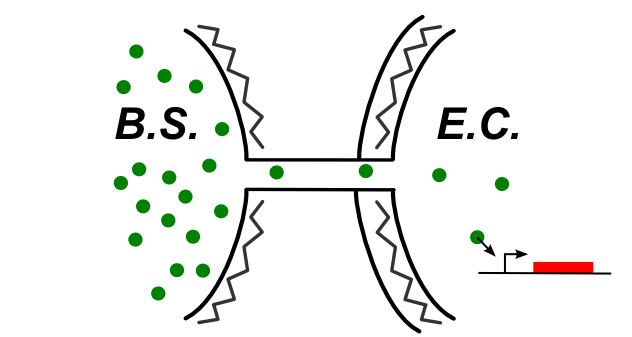
In this case, be can re-use most of the designs of the first section (see the compatibility column). We also have proposed this design:
- Xis protein diffusion: Xis is a small partner of an excisase. The latter will excise a stop codon on the DNA strand that is preventing the expression of the GFP. We had no time to build this design, but the idea is summed up on the linked page.
Design for B.subtilis / E. coli if the connection happens with the periplasm
In case the communication happens with the periplasm, we have to think about molecules that can be then transported into the cytoplasm.

We had to design more sophisticated approaches. The ideas are the following:
- MBP diffusion: we need a CRP+, MBP- E.coli mutant. We produce the MBP protein in B.subtilis and make it diffuse through the nanotubes. As long as the MBP has not reached the periplasm of E.coli, the cell cannot digest the maltose in the medium. The indirect induction of MalR by MBP triggers the expression of the GFP reporter.
Can we conclude on these designs?
If one of these designs works, it will be proved that the inter-species nanotube communication exists and this will eventually show location of the connection with the membrane. There was limited time to test these designs but we propose them for future iGEM teams to investigate.
 "
"


