Team:Paris Bettencourt/Designs
From 2011.igem.org
m (Orthograph) |
|||
| Line 4: | Line 4: | ||
<h2>Introduction</h2> | <h2>Introduction</h2> | ||
| - | <p>Our goal for this summer is characterizing the exchange of proteins through nanotubes as best as we can. We proposed two different experiments. We first wanted to <em>re-do the experiments from the original paper</em>. | + | <p>Our goal for this summer is characterizing the exchange of proteins through nanotubes as best as we can. We proposed two different experiments. We first wanted to <em>re-do the experiments from the original paper</em>. We then tried to use <em>signal amplification</em> to enhance our results and give new evidence for a non-specific cell-to-cell communication channel. To do that, we relied on two kinds of designs: <em> brand-new devices</em> and already existing <em>bi-stable switches</em>.</p> |
| - | <p>We had neither direct access to an electronic microscope nor the time to do any electronic microscopy. Our experiments therefore can only support the existence of a non-specific cell-to-cell communication channel which is strongly suspected to be the nanotubes described by Dubey and Ben-Yehuda.</p> | + | <p>We had neither direct access to an electronic microscope nor the time to do any electronic microscopy. Our experiments therefore can only support the existence of a non-specific cell-to-cell communication channel which is strongly suspected to be the nanotubes described by Dubey and Ben-Yehuda(2011).</p> |
| Line 15: | Line 15: | ||
| - | <p>In the paper from Dubey and Ben Yehuda, many types of experiments were done to demonstrate the existence and the efficiency of the nanotube network as a | + | <p>In the paper from Dubey and Ben Yehuda, many types of experiments were done to demonstrate the existence and the efficiency of the nanotube network as a mean of communication between bacteria. We aimed at reproducing these experiments in their paper and further validating the existence of nanotubes using synthetic biology. This will go a long way to clear the air on the much debated existence of the nanotube method of bacterial cell-cell communication.</p> |
<p>We observe directly or indirectly the molecules that have passed through the nanotubes.</p> | <p>We observe directly or indirectly the molecules that have passed through the nanotubes.</p> | ||
| Line 21: | Line 21: | ||
<p>The following are the experimental designs:</p> | <p>The following are the experimental designs:</p> | ||
<ul> | <ul> | ||
| - | <li><em><a href="https://2011.igem.org/Team:Paris_Bettencourt/GFP_diff">The GFP diffusion experiment:</a></em> This experiment is the keystone experiment of the paper. One strain expressing GFP is mixed with a wild type strain, and the GFP proteins are | + | <li><em><a href="https://2011.igem.org/Team:Paris_Bettencourt/GFP_diff">The GFP diffusion experiment:</a></em> This experiment is the keystone experiment of the paper. One strain expressing GFP is mixed with a wild type strain, and the GFP proteins are diffusing from the GFP+ cells to the wild-type cells.</li> |
<li><em><a href="https://2011.igem.org/Team:Paris_Bettencourt/Atb_exp">The antibiotics resistance exchange experiment:</a></em> This experiment is the most controversial experiment of the paper. The authors claim that a mix of two strains, each of them holding a resistance for a given antibiotic, can survive together in a medium containing the two antibiotics because they exchange resistance enzymes through the nanotubes. We found from our experiments that there are other possible explanations for this experiment than the existence of the nanotubes.</li> | <li><em><a href="https://2011.igem.org/Team:Paris_Bettencourt/Atb_exp">The antibiotics resistance exchange experiment:</a></em> This experiment is the most controversial experiment of the paper. The authors claim that a mix of two strains, each of them holding a resistance for a given antibiotic, can survive together in a medium containing the two antibiotics because they exchange resistance enzymes through the nanotubes. We found from our experiments that there are other possible explanations for this experiment than the existence of the nanotubes.</li> | ||
| Line 44: | Line 44: | ||
| - | <p>The problem is that each design needs a different couple of signal | + | <p>The problem is that each design needs a different couple of signal emitter/receptor. However, even with good models, the response time cannot be predicted easily because little is known about the transfer through the nanotubes. We tried to mitigate the impact of this issue by the following measures:</p> |
<p> | <p> | ||
| Line 61: | Line 61: | ||
<h2>Brand new devices</h2> | <h2>Brand new devices</h2> | ||
| - | <p>We created our first designs by designing <em>brand new devices</em> for <i>B.subtilis</i>. These BioBricks can be anything from a new promoter to a complete positive feedback looop. As discussed earlier, the goal is to pass several types of molecules, with different sizes, and see if we can observe an increase in the response time with the size of the molecule. The molecules chosen cover 3 | + | <p>We created our first designs by designing <em>brand new devices</em> for <i>B.subtilis</i>. These BioBricks can be anything from a new promoter to a complete positive feedback looop. As discussed earlier, the goal is to pass several types of molecules, with different sizes, and see if we can observe an increase in the response time with the size of the molecule. The molecules chosen cover 3 orders of magnitude of size. They are classified from the biggest to the smallest. The major systems which received much attention during the summer are:</p> |
<ul> | <ul> | ||
<li><em><a href="https://2011.igem.org/Team:Paris_Bettencourt/GFPLac_diffusion">The YFP-TetR/TetO array experiment:</a></em> This experiment is an improvement of the previous one. To observe significant fluorescence in the neighboring cell, lots of molecules have to pass through the tubes. Using the affinity of the TetR for the TetO array, we want to concentrate at one point the YFP molecules to better observe this diffusion.</li> | <li><em><a href="https://2011.igem.org/Team:Paris_Bettencourt/GFPLac_diffusion">The YFP-TetR/TetO array experiment:</a></em> This experiment is an improvement of the previous one. To observe significant fluorescence in the neighboring cell, lots of molecules have to pass through the tubes. Using the affinity of the TetR for the TetO array, we want to concentrate at one point the YFP molecules to better observe this diffusion.</li> | ||
<li><em><a href="https://2011.igem.org/Team:Paris_Bettencourt/T7_diffusion">T7 polymerase diffusion:</a></em> The T7 RNA polymerase is the RNA polymerase of the T7 phage. This is a big molecule, that recognizes a very specific promoter orthogonal to <i>B.subtilis</i>.</li> | <li><em><a href="https://2011.igem.org/Team:Paris_Bettencourt/T7_diffusion">T7 polymerase diffusion:</a></em> The T7 RNA polymerase is the RNA polymerase of the T7 phage. This is a big molecule, that recognizes a very specific promoter orthogonal to <i>B.subtilis</i>.</li> | ||
| - | <li><em><a href="https://2011.igem.org/Team:Paris_Bettencourt/tRNA_diffusion">Amber suppressor tRNA diffusion:</a></em> The principle of this design is to produce in one cell | + | <li><em><a href="https://2011.igem.org/Team:Paris_Bettencourt/tRNA_diffusion">Amber suppressor tRNA diffusion:</a></em> The principle of this design is to produce in one cell an amber supressor tRNA that will diffuse through the nanotubes. The receptor cell holds the gene for T7 with amber stop codons that cannot be translated into a functional protein as long as the tRNA amber suppressor is not present in the cell. Once expressed, the functional amber T7 RNA polymerase will trigger the T7 amplification system.</li> |
</ul> | </ul> | ||
<h2>Using bistable switches</h2> | <h2>Using bistable switches</h2> | ||
| Line 125: | Line 125: | ||
<h2>Can we conclude on these designs?</h2> | <h2>Can we conclude on these designs?</h2> | ||
| - | <p>If one of these designs works, it will be proved that the inter-species nanotube communication exists and this will eventually show location of the connection with the membrane. There was limited time to test these designs but we propose | + | <p>If one of these designs works, it will be proved that the inter-species nanotube communication exists and this will eventually show location of the connection with the membrane. There was limited time to test these designs but we propose them for future iGEM teams to investigate.</p> |
<br> | <br> | ||
Revision as of 15:31, 21 September 2011

Designs overview
Introduction
Our goal for this summer is characterizing the exchange of proteins through nanotubes as best as we can. We proposed two different experiments. We first wanted to re-do the experiments from the original paper. We then tried to use signal amplification to enhance our results and give new evidence for a non-specific cell-to-cell communication channel. To do that, we relied on two kinds of designs: brand-new devices and already existing bi-stable switches.
We had neither direct access to an electronic microscope nor the time to do any electronic microscopy. Our experiments therefore can only support the existence of a non-specific cell-to-cell communication channel which is strongly suspected to be the nanotubes described by Dubey and Ben-Yehuda(2011).
Re-doing the experiments from the original paper
In the paper from Dubey and Ben Yehuda, many types of experiments were done to demonstrate the existence and the efficiency of the nanotube network as a mean of communication between bacteria. We aimed at reproducing these experiments in their paper and further validating the existence of nanotubes using synthetic biology. This will go a long way to clear the air on the much debated existence of the nanotube method of bacterial cell-cell communication.
We observe directly or indirectly the molecules that have passed through the nanotubes.
The following are the experimental designs:
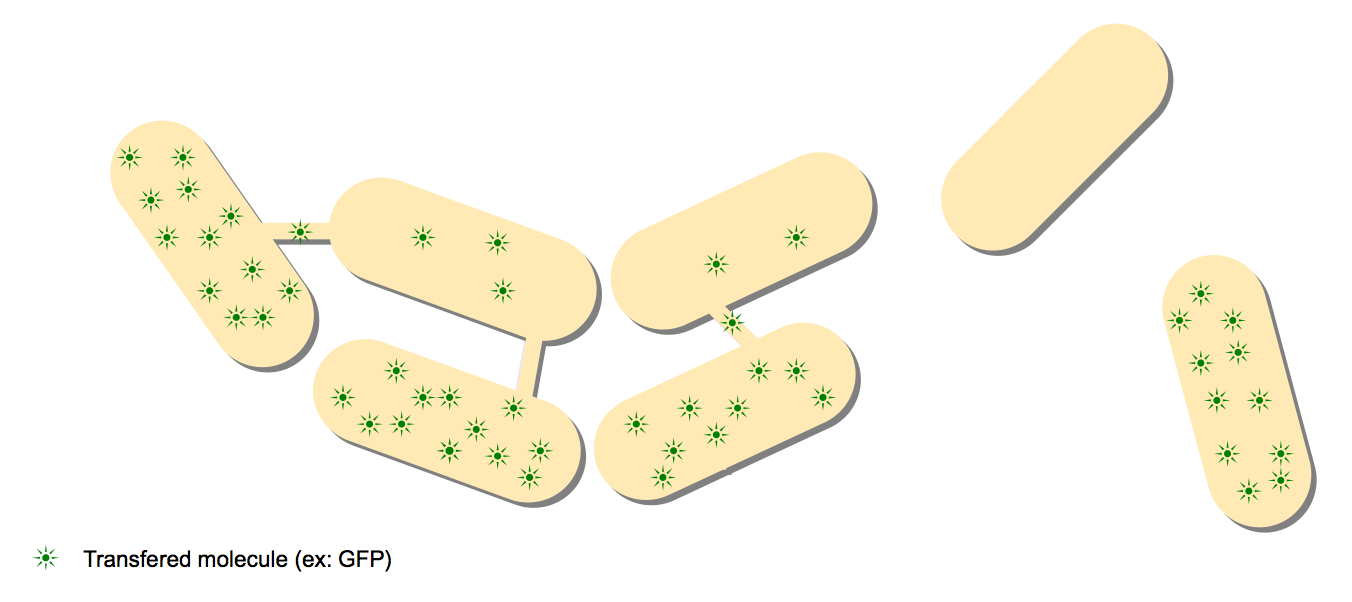
- The GFP diffusion experiment: This experiment is the keystone experiment of the paper. One strain expressing GFP is mixed with a wild type strain, and the GFP proteins are diffusing from the GFP+ cells to the wild-type cells.
- The antibiotics resistance exchange experiment: This experiment is the most controversial experiment of the paper. The authors claim that a mix of two strains, each of them holding a resistance for a given antibiotic, can survive together in a medium containing the two antibiotics because they exchange resistance enzymes through the nanotubes. We found from our experiments that there are other possible explanations for this experiment than the existence of the nanotubes.
Key specification of our designs
The aim of our project is to characterize and try to control the cellular communication through the nanotubes by using the techniques of synthetic biology. We wanted to build artificial systems to explore more deeply the properties of cells.
The first question about nanotubes we ask is what kind of molecules can pass through the nanotubes? Thus the size range of the molecules that can pass. The paper shows that GFP, calcein and plasmids can pass, but what if the molecule is bigger?
We then wondered at the transfer process in the tube. Is it simple diffusion or an active process?
We started designing the project with these two questions in mind. Using several molecules of different sizes, from a T7 polymerase to a tRNA amber supressor, we aim to test the capability and size limit for the molecules that can pass through the tubes. But, if the diffusion is passive, the speed of diffusion should be proportional to the diffusion coefficient D, that is to say inversely proportional to the radius of the object. Can we design a fast-responding system that would allow us to measure this time?
We decided that our constructs should follow the global idea summed up in the following picture:
The problem is that each design needs a different couple of signal emitter/receptor. However, even with good models, the response time cannot be predicted easily because little is known about the transfer through the nanotubes. We tried to mitigate the impact of this issue by the following measures:
- Each time the same actuator and the same monitor was used to have comparable response times in different systems.
- The relevant measure is not the response time itself, but the increase of response time between the same strains (where all of the construct emitter and receptor is in one strain) and the emitter strain/receptor in different strains.
In B.subtilis, nanotubes are reported to be formed by the cytoplasm. We had to find a molecule that can be expressed in the first cell that can trigger a genetic circuit in the second cell. The image below illustrates the idea:

Brand new devices
We created our first designs by designing brand new devices for B.subtilis. These BioBricks can be anything from a new promoter to a complete positive feedback looop. As discussed earlier, the goal is to pass several types of molecules, with different sizes, and see if we can observe an increase in the response time with the size of the molecule. The molecules chosen cover 3 orders of magnitude of size. They are classified from the biggest to the smallest. The major systems which received much attention during the summer are:
- The YFP-TetR/TetO array experiment: This experiment is an improvement of the previous one. To observe significant fluorescence in the neighboring cell, lots of molecules have to pass through the tubes. Using the affinity of the TetR for the TetO array, we want to concentrate at one point the YFP molecules to better observe this diffusion.
- T7 polymerase diffusion: The T7 RNA polymerase is the RNA polymerase of the T7 phage. This is a big molecule, that recognizes a very specific promoter orthogonal to B.subtilis.
- Amber suppressor tRNA diffusion: The principle of this design is to produce in one cell an amber supressor tRNA that will diffuse through the nanotubes. The receptor cell holds the gene for T7 with amber stop codons that cannot be translated into a functional protein as long as the tRNA amber suppressor is not present in the cell. Once expressed, the functional amber T7 RNA polymerase will trigger the T7 amplification system.
Using bistable switches
We quickly noticed that we could take advantage of bistable switches already existing in B.subtilis. Some of these switches are natural (such as the Sin operon), some are state-of-the-art synthetic biology products (the push-on/push-off system). We want to try to diffuse through the nanotubes a molecule that will toggle the switch from one position to another. Here is the list of designs we tried:
- ComS diffusion: ComS is an inhibitor of the MecA protease in B.subtilis. It plays a key role in the triggering of the competence and sporulation mechanisms. The idea is to trigger the switch of the MeKS system of the receptor cell by diffusing ComS through the nanotubes. A major precaution is to prevent the first cell from sporulating.
- Sin operon:
- Lambda switch:
Designs we thought of & inter-species communication
We neither had time nor the manpower to try all the ideas we had. Some designs too complicated to be feasible. We also wanted to investigate at length the Subtilis/Coli connexions but had no time to do it properly. We nonetheless present all our ideas in this section.
Abandonned design for B.subtilis
- Xis protein diffusion: Xis is a small partner of an excisase. The latter will excise a stop codon on the DNA strand that is preventing the expression of the GFP. We had no time to build this design, but the idea is summed up on the linked page.
Designs for B.subtilis-E.coli communication
The article demonstrates that nanotubes established inside the B.subtilis family can also be formed between B.subtilis and E.coli. These two types of bacteria are really different since one is Gram+ and the other is Gram-. Their membrane is really different since there is no periplasm in B.subtilis. The existence of inter-species nanotubes did really spike our interest and we also created designs to test this aspect of the nanotube communication. We had few time to test these ideas but we present here the designs we thought of. For all the designs which are simply the ones for B.subtilis applied to a Subtilis/Coli connexion, we plan to experiment them in the future since they require very little additional work.
B.subtilis is Gram- so the nanotubes seem to create a link from the cytoplasm of the first cell to the cytoplasm of the second cell. In the case of the Gram+ bacteria, like E.coli, we wonder which membrane forms the nanotubes . Do they create a link with the periplasm, or with the cytoplasm? To test the two hypotheses, we tried to create a design for each of the two possibilities. If one work and the other does not, the answer to this question will be known.
If nanotube communication can be established between E.coli and B.subtilis, it is not known whether the communication happens through the cytoplasm or the periplasm. To test these two hypotheses, two designs were made:
If communication happens with the cytoplasm
The image below sums up the kind of connection that can be established if nanotubes connect the two cytoplasms. Hence, some of the designs we have shown above are still available for B.subtilis to E.coli.

Here are the designs created in this case.
- T7 polymerase diffusion: The T7 RNA polymerase is the RNA polymerase of the T7 phage. This is a pretty big molecule, that recognizes a very specific promoter orthogonal to B.subtilis and E.coli.
- KinA diffusion: The idea is to make a complex partner to diffuse from one cell to the other in order to trigger the sporulation of the second cell, even in exponential phase.
- ComS diffusion: ComS is an inhibitor of the MecA protease in B.subtilis. It plays a key role in the triggering of the competence and sporulation mechanisms. The idea is to trigger the switch of the MeKS system of the receptor cell by diffusing ComS through the nanotubes. A major precaution is to prevent the first cell from sporulating.
- Amber suppressor tRNA diffusion: The principle of this design is to produce in one cell an amber suppressor tRNA that will diffuse through the nanotubes. The receptor cell holds the gene for a T7 with amber stop codons that cannot be translated into a functional protein as long as the tRNA amber suppressor is not present in the cell. Once expressed, the functional amber T7 RNA polymerase will trigger the T7 amplification system.
If communication happens with the periplasm
In case the communication happens with the periplasm, we have to think about molecules that can be then transported into the cytoplasm.

We had to design more sophisticated approaches. The ideas are the following:
- MBP diffusion: we need a CRP+, MBP- E.coli mutant. We produce the MBP protein in B.subtilis and make it diffuse through the nanotubes. As long as the MBP has not reached the periplasm of E.coli, the cell cannot digest the maltose in the medium. The indirect induction of MalR by MBP triggers the expression of the GFP reporter.
- OmpR diffusion: we need a OmpR- Receptor* E.coli mutant. We produce the OmpR protein in B.subtilis. As long as the OmpR has not diffused from B.subtilis, the signaling cascade cannot be activated. With the rescue by B.subtilis of the OmpR protein, the expression of the reporter gene is activated.
Can we conclude on these designs?
If one of these designs works, it will be proved that the inter-species nanotube communication exists and this will eventually show location of the connection with the membrane. There was limited time to test these designs but we propose them for future iGEM teams to investigate.
 "
"