Team:EPF-Lausanne/Our Project/T7 promoter variants/recovery
From 2011.igem.org
(→Results) |
|||
| Line 2: | Line 2: | ||
| - | + | == Introduction == | |
We now wanted to find out if cells release their plasmid DNA into the culture supernatant when they lyse and if we can recover this DNA for further analyses. | We now wanted to find out if cells release their plasmid DNA into the culture supernatant when they lyse and if we can recover this DNA for further analyses. | ||
| - | + | == Experimental Setup == | |
[[File:broth_noiptg.png|330px|left]] | [[File:broth_noiptg.png|330px|left]] | ||
| Line 19: | Line 19: | ||
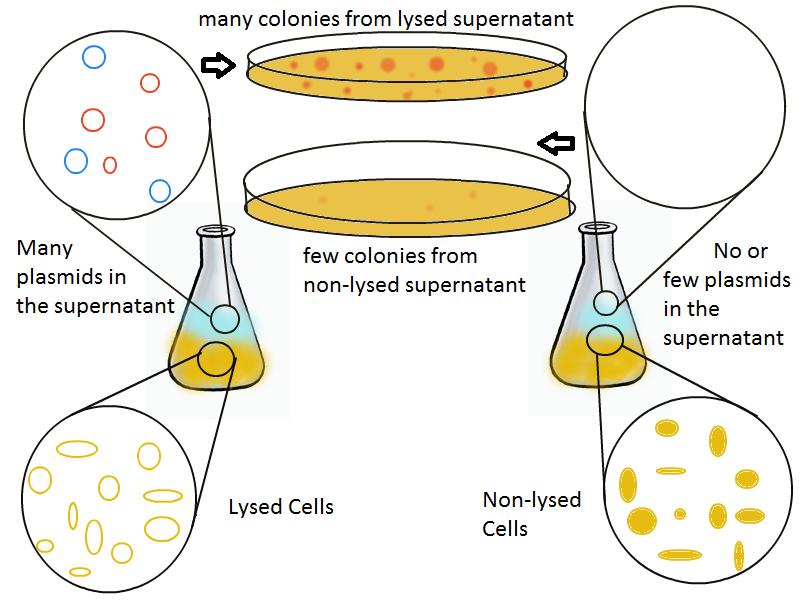
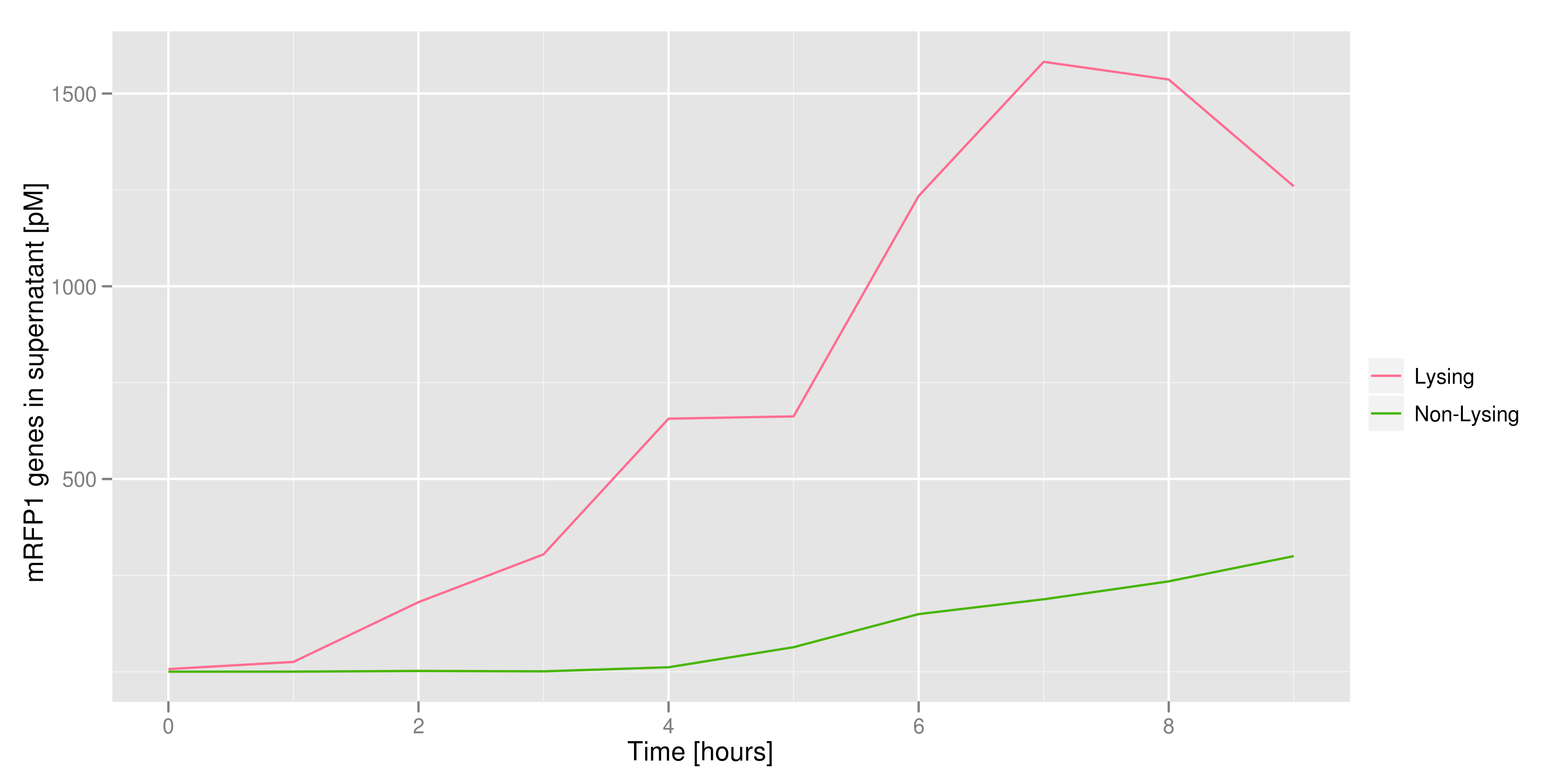
Once lysis has been induced, we harvest the supernatant every hour, centrifuge it, and sterile filter it in order to remove cell debris. With this purified supernatant, we proceed to two different methods for calculating the amount of DNA that was collected. One method uses the qPCR to amplify a particular sequence of DNA in the desired plasmid (in our case an RFP-containing plasmid). If the qPCR produces large quantities of the DNA, we can conclude that the plasmid was prominently present in the supernatant. The other method involves transforming the supernatant into competent cells and counting the number of resulting colonies. The colony count alongside the qPCR data gives a good understanding of how much DNA could be recovered from the supernatant. | Once lysis has been induced, we harvest the supernatant every hour, centrifuge it, and sterile filter it in order to remove cell debris. With this purified supernatant, we proceed to two different methods for calculating the amount of DNA that was collected. One method uses the qPCR to amplify a particular sequence of DNA in the desired plasmid (in our case an RFP-containing plasmid). If the qPCR produces large quantities of the DNA, we can conclude that the plasmid was prominently present in the supernatant. The other method involves transforming the supernatant into competent cells and counting the number of resulting colonies. The colony count alongside the qPCR data gives a good understanding of how much DNA could be recovered from the supernatant. | ||
| - | + | == Results == | |
[[File:first_DNA_supernatant.png|350px|left]] | [[File:first_DNA_supernatant.png|350px|left]] | ||
Revision as of 13:18, 21 September 2011
Lysis Selection System
Lysis selection system Main | Lysis Characterization | DNA Recovery | DNA Selection | T7 Promoter Variants
Introduction
We now wanted to find out if cells release their plasmid DNA into the culture supernatant when they lyse and if we can recover this DNA for further analyses.
Experimental Setup
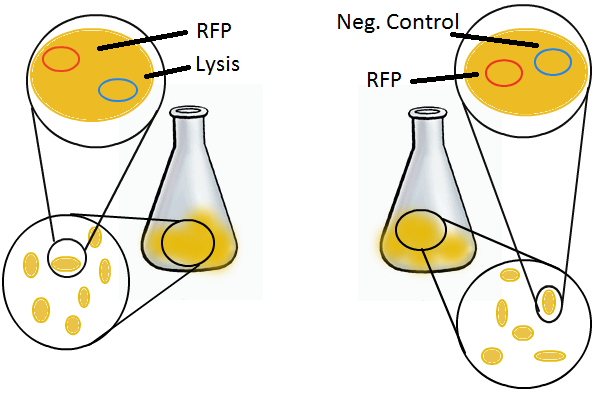
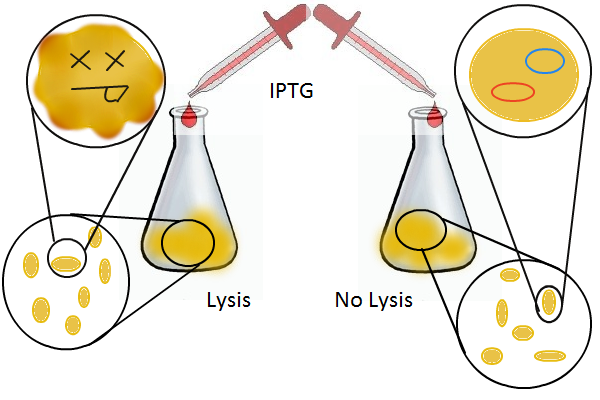
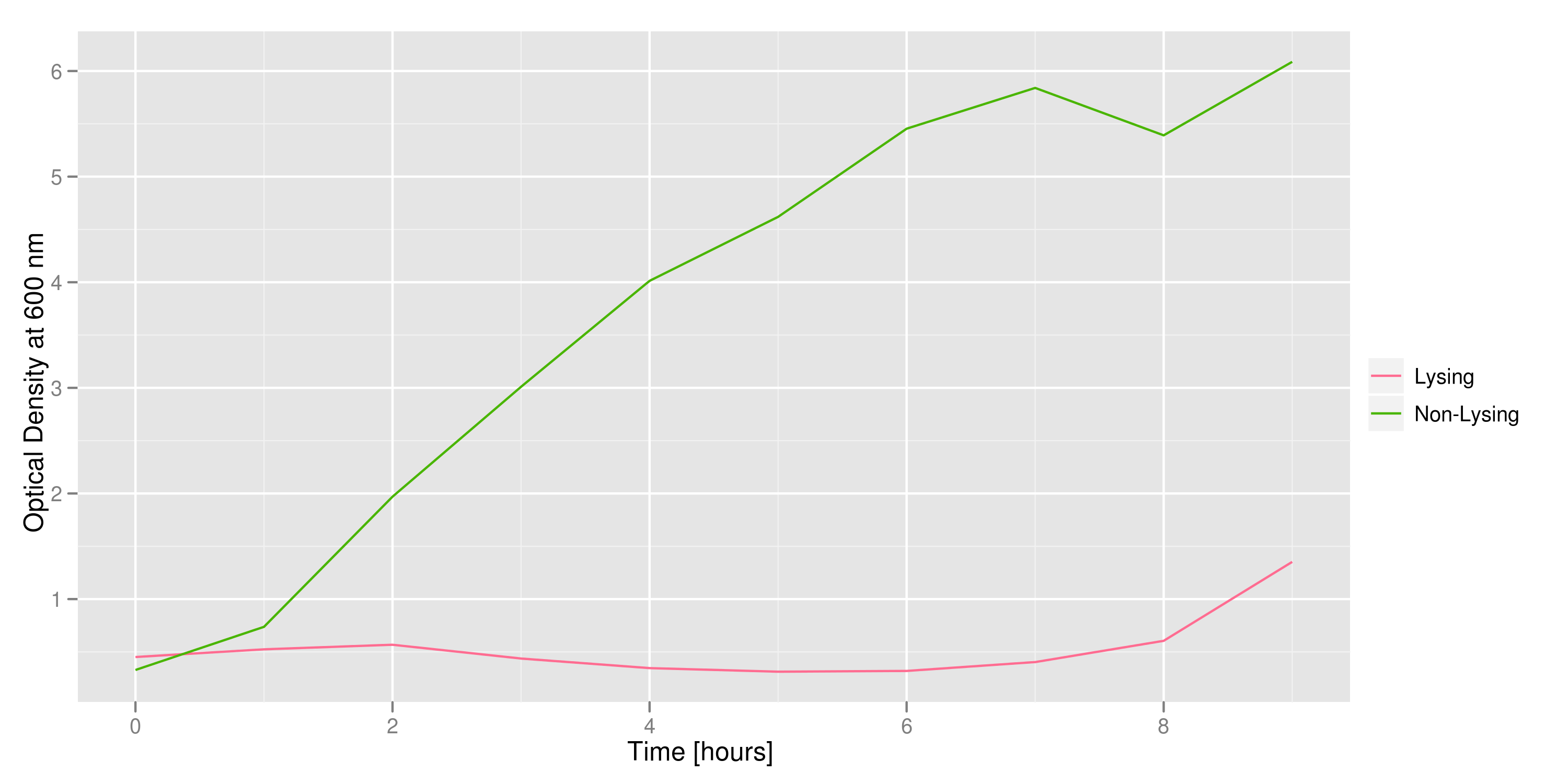
We grow two large cultures of cells. One contains cells that will lyse and release plasmids into the supernatant while the other has non-lysing, "normal" cells. Adding IPTG to both flasks induces lysis in one set of cells but not in the others. If you would like to find out more about how IPTG induction experiments work, please click here.
Once lysis has been induced, we harvest the supernatant every hour, centrifuge it, and sterile filter it in order to remove cell debris. With this purified supernatant, we proceed to two different methods for calculating the amount of DNA that was collected. One method uses the qPCR to amplify a particular sequence of DNA in the desired plasmid (in our case an RFP-containing plasmid). If the qPCR produces large quantities of the DNA, we can conclude that the plasmid was prominently present in the supernatant. The other method involves transforming the supernatant into competent cells and counting the number of resulting colonies. The colony count alongside the qPCR data gives a good understanding of how much DNA could be recovered from the supernatant.
Results
 "
"