Team:Paris Liliane Bettencourt/Notebook/2011/09/08/
From 2011.igem.org
(→Digestion and gel) |
|||
| Line 35: | Line 35: | ||
Miniprep of the 3 last clones of YFP-TetR BB (that were not sequenced or red) | Miniprep of the 3 last clones of YFP-TetR BB (that were not sequenced or red) | ||
| + | |||
| + | |||
| + | == Hovannes-Baptiste == | ||
| + | |||
| + | === Preparation of slides === | ||
| + | |||
| + | Dilution of overnight cultures : PY79 (gfp-), PY79 (gfp+), 3610 (noted 3610 gfp-) and 3610 with GPF (noted 3610 gfp+) . <br> | ||
| + | |||
| + | We tested quicly PY79+S12 and saw that once again there was no fluorescence. We will have to see if our -80°C glycerol for this strain is still ok. However 3610 strains arevery quick and exhibited a strong fluorescence. We will continue to test them a lot during the following days. | ||
| + | |||
| + | We waited to an OD of 0.4 (600 nm). | ||
| + | |||
| + | Two well slides : | ||
| + | * 1-control (PY79 only) 2-Mix (PY79 and 3610 gfp+) | ||
| + | * 1-control (3610 gfp-) 2-Mix (3610 gfp- and 3610 gfp+) | ||
| + | <br> | ||
| + | |||
| + | === Observation === | ||
| + | -37°C Microscopy-<br> | ||
| + | |||
| + | We observed the plate with TRANS and YFP-filter settings on the old Zeiss microscope. The 3610 gfp+ strain proved to exhibit a strong fluorescence in both cases. We had two experiments of approximately 3 hours which gave the same results. The images were better than what we used to do, but the cells were not concentrated enough (verry fare from what we saw in the Ben-Yahuda article). We did not see any evidence of nanotubes probably because of this. We will try to concentrate the cells more this week-end. | ||
| + | |||
| + | [[File:0907_trans_PY79_PY79S12.jpg|450px|thumb|center|TRANS at t=0min]] | ||
| + | [[File:0907_fluo_PY79_PY79S12.jpg|450px|thumb|center|GFP at t=0min]] | ||
| + | |||
| + | |||
| + | We followed the plate a few hours nonetheless but no result was visible (the florescent cell divided only 2 times). | ||
| + | |||
| + | |||
| + | |||
| + | <html> | ||
| + | <!-- PAGE FOOTER -- ITEMS FROM COLUMN ! HAVE BEEN MOVED HERE -- RDR --> | ||
| + | |||
| + | <div id="footer-wrapper"> | ||
| + | <div id="footer"> | ||
| + | <div id="f-poweredbyico"><a href="http://www.mediawiki.org/"><img src="/wiki/skins/common/images/poweredby_mediawiki_88x31.png" height="31" width="88" alt="Powered by MediaWiki" /></a></div> <div id="f-copyrightico"><a href="http://creativecommons.org/licenses/by/3.0/"><img src="http://i.creativecommons.org/l/by/3.0/88x31.png" alt="Attribution 3.0 Unported" width="88" height="31" /></a></div> <ul id="f-list"> | ||
| + | |||
| + | |||
| + | <!-- Recentchanges is not handles well DEBUG --> | ||
| + | <li id="t-recentchanges"><a href="/Special:RecentChanges" | ||
| + | title='Recent changes'>Recent changes</a></li> | ||
| + | |||
| + | <li id="t-whatlinkshere"><a href="/Special:WhatLinksHere/Team:Paris_Bettencourt/Modeling" | ||
| + | title="List of all wiki pages that link here [j]" accesskey="j">What links here</a></li> | ||
| + | |||
| + | <li id="t-recentchangeslinked"><a href="/Special:RecentChangesLinked/Team:Paris_Bettencourt/Modeling" | ||
| + | title="Recent changes in pages linked from this page [k]" accesskey="k">Related changes</a></li> | ||
| + | |||
| + | |||
| + | |||
| + | <li id="t-upload"><a href="/Special:Upload" | ||
| + | title="Upload files [u]" accesskey="u">Upload file</a> | ||
| + | </li> | ||
| + | <li id="t-specialpages"><a href="/Special:SpecialPages" | ||
| + | title="List of all special pages [q]" accesskey="q">Special pages</a> | ||
| + | |||
| + | </li> | ||
| + | <li><a href='/Special:Preferences'>My preferences</a></li> | ||
| + | </ul> | ||
| + | </div> <!-- close footer --> | ||
| + | <div id='footer'> | ||
| + | <ul id="f-list"> | ||
| + | |||
| + | <li id="t-print"><a href="/wiki/index.php?title=Team:Paris_Bettencourt/Modeling&printable=yes" | ||
| + | title="Printable version of this page [p]" accesskey="p">Printable version</a> | ||
| + | |||
| + | </li> | ||
| + | |||
| + | <li id="t-permalink"><a href="/wiki/index.php?title=Team:Paris_Bettencourt/Modeling&oldid=86565" | ||
| + | title="Permanent link to this revision of the page">Permanent link</a> | ||
| + | </li> | ||
| + | |||
| + | |||
| + | <li id="privacy"><a href="/2011.igem.org:Privacy_policy" title="2011.igem.org:Privacy policy">Privacy policy</a></li> | ||
| + | <li id="disclaimer"><a href="/2011.igem.org:General_disclaimer" title="2011.igem.org:General disclaimer">Disclaimers</a></li> | ||
| + | </ul> | ||
| + | |||
| + | </div> <!-- close footer --> | ||
| + | </div> <!-- close footer-wrapper --> | ||
| + | </div> | ||
| + | </html> | ||
Revision as of 14:16, 12 September 2011

Contents |
Cyrille
Digestion and gel
S24 is prepared to be cloned with
- RFP - TT
- T7 amber - TT
- ComS
- KinA-TT
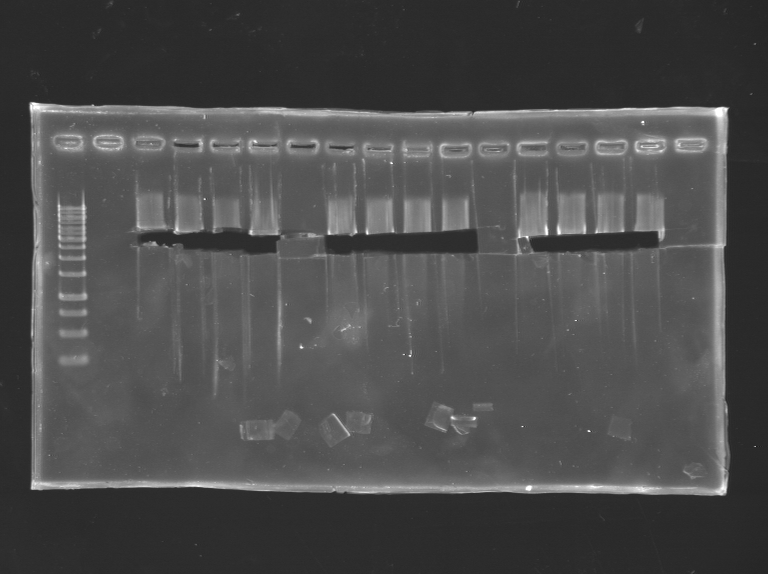
3*4 500ng of S24 was digested and runned on the gel The insert was digested several time.
Then the gel was runned and the bands cutted.
Then I proceed to a gel extraction of the bands.
PCR colony of TetO/TetR
New attempt of PCR colony to find one good clone. Runned on a gel
Miniprep of YFP-TetR BB
Miniprep of the 3 last clones of YFP-TetR BB (that were not sequenced or red)
Hovannes-Baptiste
Preparation of slides
Dilution of overnight cultures : PY79 (gfp-), PY79 (gfp+), 3610 (noted 3610 gfp-) and 3610 with GPF (noted 3610 gfp+) .
We tested quicly PY79+S12 and saw that once again there was no fluorescence. We will have to see if our -80°C glycerol for this strain is still ok. However 3610 strains arevery quick and exhibited a strong fluorescence. We will continue to test them a lot during the following days.
We waited to an OD of 0.4 (600 nm).
Two well slides :
- 1-control (PY79 only) 2-Mix (PY79 and 3610 gfp+)
- 1-control (3610 gfp-) 2-Mix (3610 gfp- and 3610 gfp+)
Observation
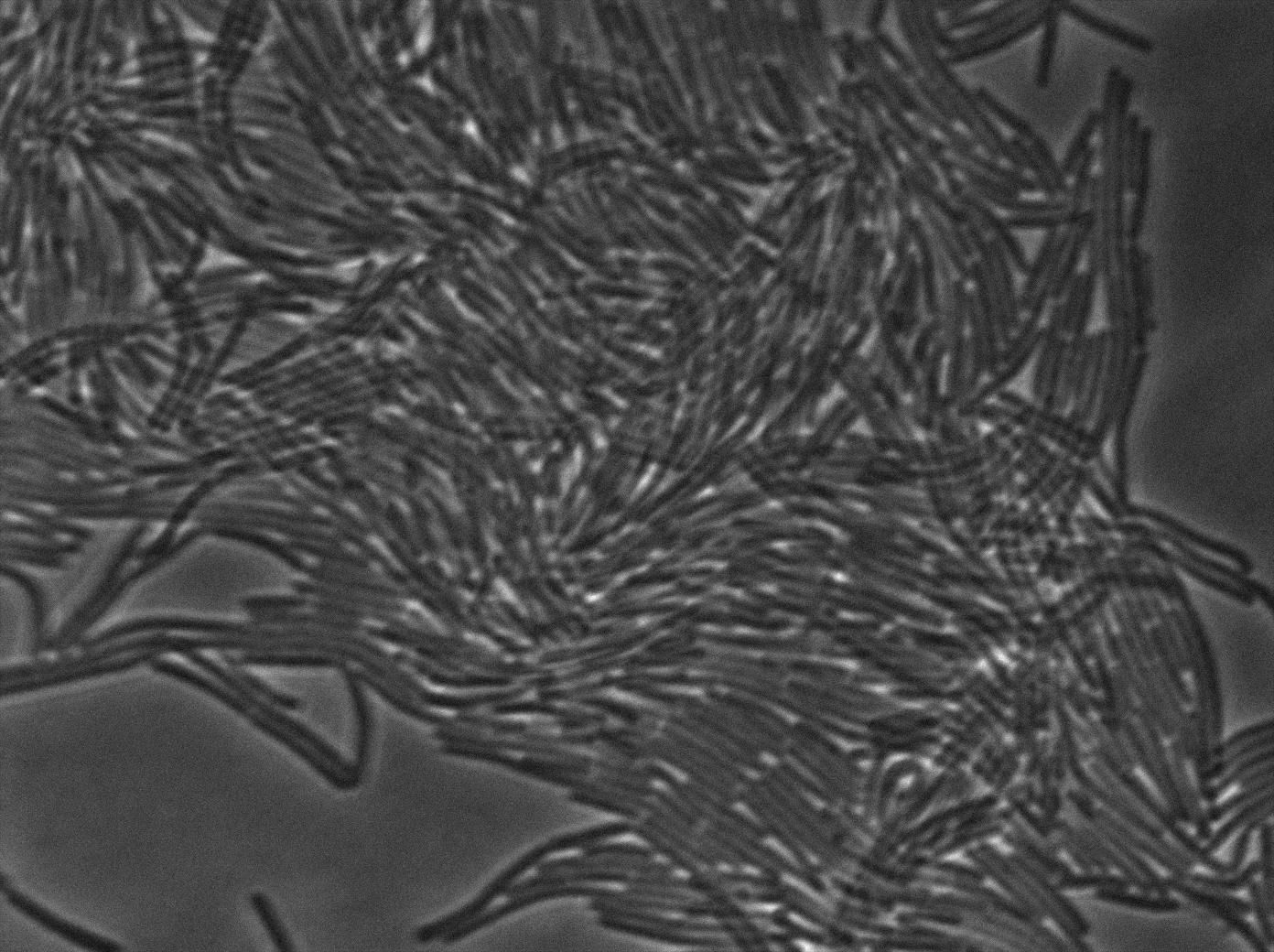
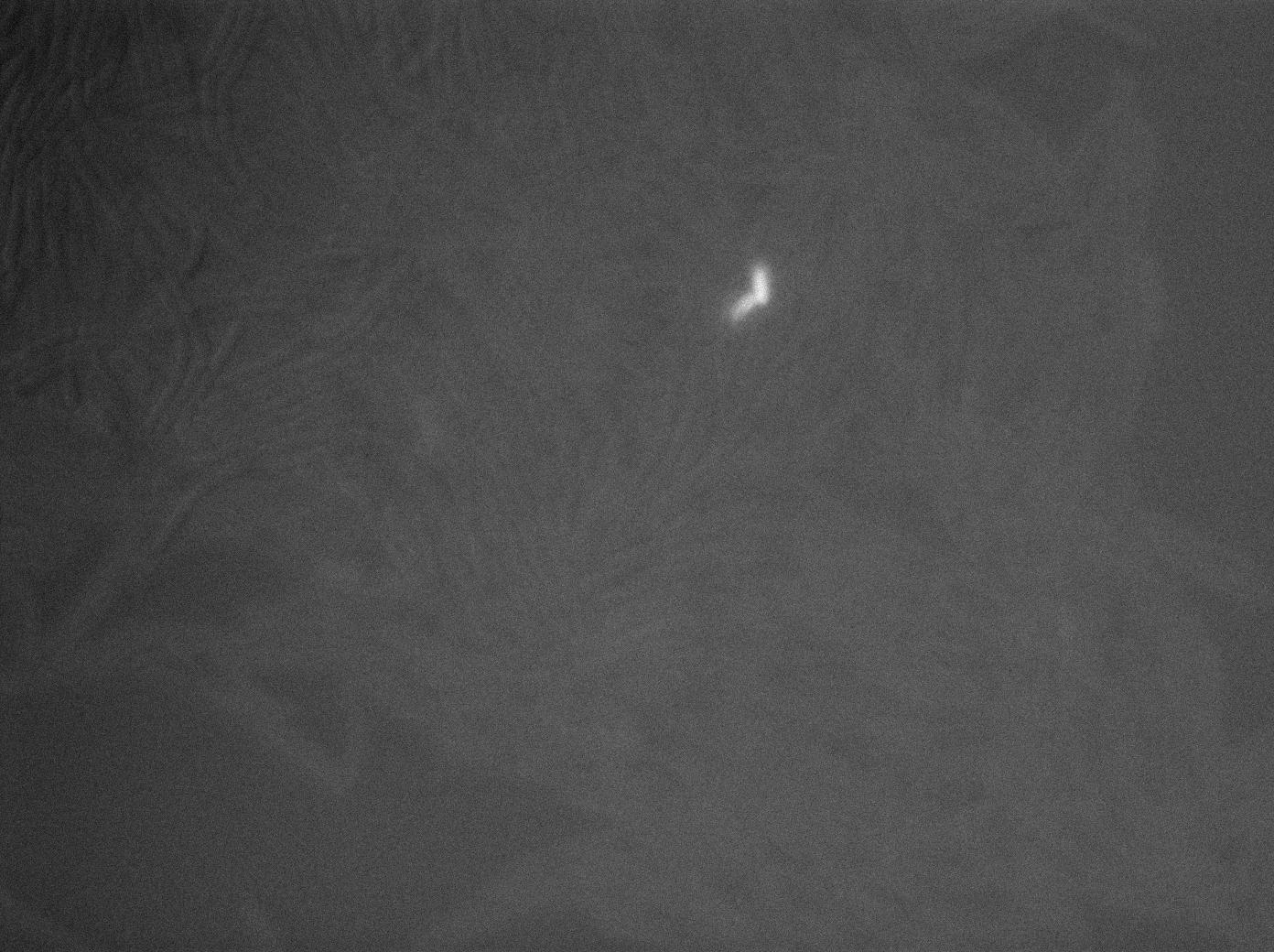
-37°C Microscopy-
We observed the plate with TRANS and YFP-filter settings on the old Zeiss microscope. The 3610 gfp+ strain proved to exhibit a strong fluorescence in both cases. We had two experiments of approximately 3 hours which gave the same results. The images were better than what we used to do, but the cells were not concentrated enough (verry fare from what we saw in the Ben-Yahuda article). We did not see any evidence of nanotubes probably because of this. We will try to concentrate the cells more this week-end.
We followed the plate a few hours nonetheless but no result was visible (the florescent cell divided only 2 times).
 "
"