Team:EPF-Lausanne/Protocols/IPTG test
From 2011.igem.org
(Difference between revisions)
| Line 3: | Line 3: | ||
[[File:EPFL2011_IPTG.png|500px]] | [[File:EPFL2011_IPTG.png|500px]] | ||
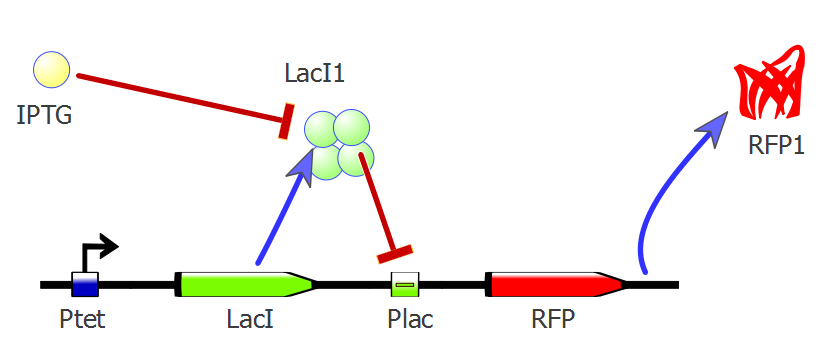
| - | IPTG is a molecular mimic for allolactose | + | IPTG is a molecular mimic for allolactose which binds to LacI and inhibiths it. LacI cannot bind to Plac and repress expression any more, therefore IPTG allows the expression of the gene under the control of this promoter (RFP or T4 lysis cassette in our case). |
| - | * shake gently an overnight | + | * shake gently an overnight culture of bacteria to resuspend cells (you can do it by inverting the tubes until the solution is homogeneous) |
* take an aliquot of 100 microL and transfer to a new eppendorf tube | * take an aliquot of 100 microL and transfer to a new eppendorf tube | ||
* add 900 microL of new medium (LB + antibiotic); this will be a dilution 1:10 | * add 900 microL of new medium (LB + antibiotic); this will be a dilution 1:10 | ||
Latest revision as of 09:44, 18 August 2011
IPTG test
Back to protocols.IPTG is a molecular mimic for allolactose which binds to LacI and inhibiths it. LacI cannot bind to Plac and repress expression any more, therefore IPTG allows the expression of the gene under the control of this promoter (RFP or T4 lysis cassette in our case).
- shake gently an overnight culture of bacteria to resuspend cells (you can do it by inverting the tubes until the solution is homogeneous)
- take an aliquot of 100 microL and transfer to a new eppendorf tube
- add 900 microL of new medium (LB + antibiotic); this will be a dilution 1:10
- spot the cells (100 microL) in a multi-well: 2 wells per colony (the second is the negative control)
- for every colony: in one well add IPTG (500 microM) and in the second well add the same volume of water (negative control)
- cover each well with 20 microL of mineral oil (Chill-Out Liquid Wax)
Move to the plate-reader and remind to start it up 10' before starting
- open the software Gen5
- use the old file from IGEM but remember to save it as a new file (in order to be able to modify it without deleting the old settings)
- double click on procedure
- set temp at 37°C
- in "start kinetic" set:
- time: 12 hours
- interval: 10 minutes
- read (for the OD): keep the settings like that (absorbance, endpoint, normal) and put 600 nm as wavelength
- read (for the RFP): keep the settings like that (endpoint, normal) except for "absorbance" that has to be changed in "fluorescence"
- for excitation wavelength put 585 nm and 8 as range
- for emission wavelength put 615 nm and 9 as range
- optic position: bottom
- sensivity: 80 max and 50 min
- when you have finished the setup click the last icon in the upper bar ang to to "Pre-heating" setting it to 37°C
- open the lid of the instrument and place the multi-well plate (remember to clean beneath it to clean all the dust)
- click the next-to-last button in the upper bar to start
- when it's over remember to:
- trash the plate in the yellow bag and
- set Off the pre-heating
- switch the plate-reader off
 "
"