Team:Freiburg/Notebook/15 July
From 2011.igem.org
(Difference between revisions)
(→Lysis cassette) |
|||
| (19 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | {{:Team:Freiburg/Templates/header}} | ||
| + | <html> | ||
| + | <div id="notebook-page-header"> | ||
| + | <div id="notebook-back" width="100px" > | ||
| + | <a href="https://2011.igem.org/Team:Freiburg/Notebook/14_July">Previous entry</a> | ||
| + | </div> | ||
| + | <div id="notebook-title"> | ||
| + | <a href="https://2011.igem.org/Team:Freiburg/Notebook"> 15 July </a> | ||
| + | </div> | ||
| + | <div id="notebook-next"> | ||
| + | <a href="https://2011.igem.org/Team:Freiburg/Notebook/16_July">Next entry</a> | ||
| + | </div> | ||
| + | </div> | ||
| + | </html> | ||
| + | |||
==Meeting== | ==Meeting== | ||
attendants: Tobi, Theo, Julia, Rüdiger, Jakob, Sandra | attendants: Tobi, Theo, Julia, Rüdiger, Jakob, Sandra | ||
| - | ===<span style="color: | + | ===<span style="color:blue;">blue light receptor</span>=== |
| - | already done: | + | |
| + | already done: | ||
| + | |||
| + | # Transformation of Lov-Tap in cells. | ||
| Line 11: | Line 29: | ||
To-do: | To-do: | ||
| + | # Cloning of promotor (medium) in Lov-Tap part and perform a transformation. Plating out of the cells on trypthophan-free medium. | ||
| + | <br/> | ||
| - | === | + | ===Quick change=== |
| + | '''Investigators: Theo''' | ||
already done: | already done: | ||
| - | + | #repressor part (BBa_K098995) has no bases between RBS (B0034) and start codon (K098997), resulting in no translation. | |
| + | #*quickchange of the repressor part to insert 6bp between RBS and start codon | ||
To-do: | To-do: | ||
| - | + | #DpnI digestion to digest the DNA template (methylated DNA) of the PCR to have only the new synthesized DNA strand. | |
| + | #After digestion with DpnI, transformation of cells with the corrected part (BBa_K08995) | ||
| + | <br/> | ||
| - | + | ==<span style="color:orange;">Lysis cassette</span>== | |
| + | ===Digestion of Quickchange=== | ||
| - | + | {| style="border-spacing:0;" | |
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Name: Theo | ||
| - | |||
| - | |||
| - | + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Date: 15.07.2011 | |
| - | # | + | |
| - | + | |- | |
| + | | colspan="2" style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Continue from Experiment 14.07.2011 | ||
| + | |||
| + | Quickchange PCR | ||
| + | |||
| + | |- | ||
| + | | colspan="2" style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Project Name: Correct number of nucleotides between RBS and ATG of temp. sensitive repressor from Lysis Device | ||
| + | |||
| + | |} | ||
| + | |||
| + | {| style="border-spacing:0;" | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 4,5μl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| H<sub>2</sub>O | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 4μl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Buffer, NEB4 | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 4μl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| BSA (10x) | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 1,5 μl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Enzym 1 | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| DpnI | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 21 μl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| DNA | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Quickchange PCR | ||
| + | |||
| + | |} | ||
| + | 35 μl total volume | ||
| + | |||
| + | |||
| + | Incubate for about 1h at 37°C + Heat inactivation at 80°C for 20min. | ||
| - | |||
| - | |||
<br/> | <br/> | ||
| - | === | + | ===Transformation''' '''=== |
| - | |||
| + | {| style="border-spacing:0;" | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Name: Theo | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Date: 15.07.2011 | ||
| + | |- | ||
| + | | colspan="2" style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Continue from 15.07.2011 Digestion of Quickchange | ||
| - | + | Experiment | |
| + | |||
| + | |- | ||
| + | | colspan="2" style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Project Name: Correct number of nucleotides between RBS and ATG of temp. sensitive repressor from Lysis Device | ||
| + | |||
| + | |} | ||
| + | Procedure | ||
| + | |||
| + | |||
| + | # take cells from -80°C freezer and put them on ice! (every eppi contains about 400 μl cells) | ||
| + | # thaw cells on ice 20 minutes | ||
| + | # pipette 50 μl cells and 2 μl DNA into eppi still on ice! | ||
| + | # Incubate for 30 minutes on ice | ||
| + | # Heat at 42°C for 60 sec | ||
| + | # Incubate on ice for 5 minutes | ||
| + | # Add 200 μl LB Broth | ||
| + | # Incubate for 2 hours at 37°C (cells with lysis cassette at 30°C!!) | ||
| + | # Plate 50 μl and 200μl on two different LB/Agar plates with appropriate antibiotic resistance | ||
| + | |||
| + | '''Documentation:''' | ||
| + | |||
| + | Why are you doing this experiment? Name of the sample? Where are they stored? Name the vector with inserts, antibiotika resistance etc. | ||
| + | |||
| + | |||
| + | {| style="border-spacing:0;" | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| It was done in ordert to correct the number of nucleotides between RBS and ATG of the temperature sensitive repressor from our Lysis Device, ie to insert 6bp. | ||
| + | |||
| + | |||
| + | It didn’t work because the false DNA Template was taken (S15 - K124014 - instead of S11 - K098995 -). | ||
| + | |||
| + | |||
| + | This will be corrected by doing another Quickchange using S11 (ie K098995) as template. | ||
| + | |||
| + | |} | ||
| - | |||
<br/> | <br/> | ||
| - | ==<span style="color: | + | ==<span style="color:grey;">Precipitator</span>== |
| - | + | '''PCR''' | |
| - | |||
| - | + | {| style="border-spacing:0;" | |
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Name: | ||
| - | = | + | Ruediger |
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Date: | ||
| - | + | 18.07.2011 | |
| + | |- | ||
| + | | colspan="2" style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Continue from Experiment (Date)PCR 1507 | ||
| + | (Name) Ruediger | ||
| + | |- | ||
| + | | colspan="2" style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Project Name: | ||
| - | + | GFP Pbd | |
| - | + | |} | |
| + | PCR-Mixture for one Reaction: | ||
| - | + | For a 50 µl reaction use | |
| + | {| style="border-spacing:0;" | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 32,5µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| H<sub>2</sub>0 | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 10µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 5x Phusion Buffer | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| - | = | + | |- |
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 2.5µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Primer fw | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| - | === | + | |- |
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 2.5µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Primer dw | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| - | + | |- | |
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 1µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| dNTPs | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 1µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| DNA-Template | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| + | |- | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| 0.5 µl | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| Phusion (add in the end) | ||
| + | | style="border:0.0069in solid #00000a;padding-top:0in;padding-bottom:0in;padding-left:0.075in;padding-right:0.075in;"| | ||
| - | + | |} | |
| + | What program do you use? | ||
| + | |||
| + | 10x (95C-41/52C-72C) + 25x ((95C-60C-72C) | ||
| + | |||
| + | |||
| + | How did you label the PCR-Product, where is it stored and what do you do next? | ||
| + | |||
| + | |||
| + | Reactions: | ||
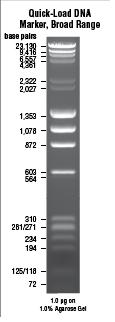
| + | |||
| + | lane 1 | ||
| + | |||
| + | quick load braod range marker | ||
| + | |||
| + | lane 2 | ||
| + | |||
| + | empty | ||
| + | |||
| + | lane 3 | ||
| + | |||
| + | P28+P18+M14.1 | ||
| + | |||
| + | lane 4 | ||
| + | |||
| + | P28+P19+M14.1 | ||
| + | |||
| + | lane 5 | ||
| + | |||
| + | P28+P20+M14.1 | ||
| + | |||
| + | |||
| + | Results: | ||
| + | |||
| + | did not work well. | ||
| + | |||
| + | Strange band size in lane 3. 4 and 5 did not work. | ||
| - | + | [[File:Freiburg11_7_16_2011_2_52_42_PM.Jpg]] | |
| + | [[File:Freiburg11 Phusion ladder.JPG]] | ||
| - | + | {{:Team:Freiburg/Templates/footer}} | |
Latest revision as of 00:56, 22 September 2011
 "
"
 Contact
Contact