Team:UT-Tokyo/Data
From 2011.igem.org
m |
|||
| (13 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:UT-Tokyo/Templates/BeginContent|fullpagename=Team:UT-Tokyo/Data|subpagename=Data}} | {{:Team:UT-Tokyo/Templates/BeginContent|fullpagename=Team:UT-Tokyo/Data|subpagename=Data}} | ||
| - | + | =How our system works= | |
| - | + | ==SMART ''E. coli''== | |
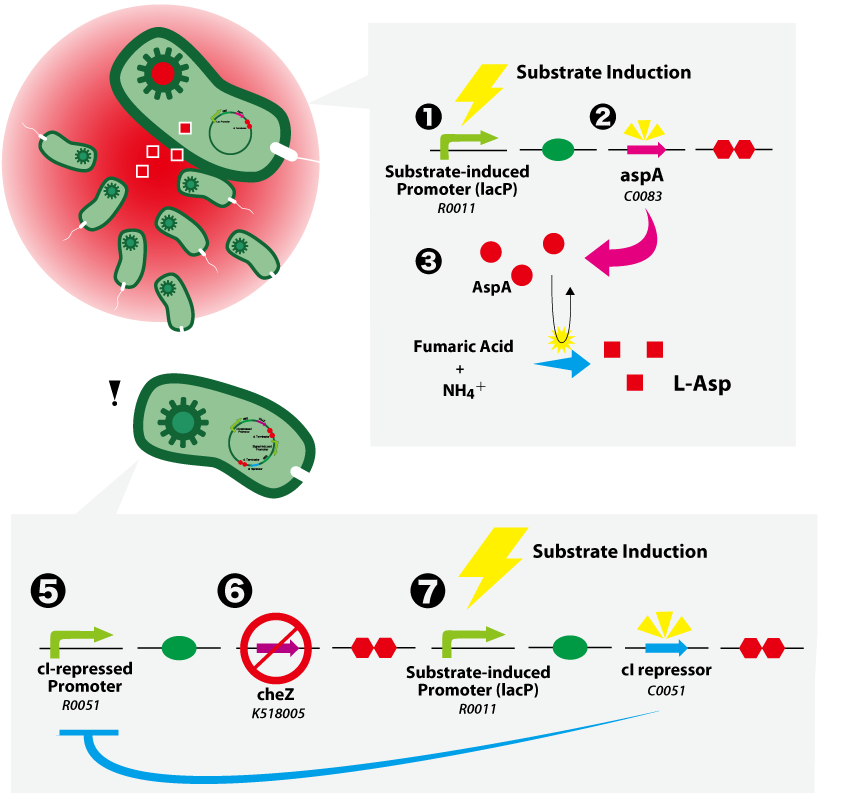
| - | {{:Team:UT-Tokyo/Templates/Image|file=UT-Tokyo_Data-Top.png|caption=Figure 1. SMART E.coli}} | + | {{:Team:UT-Tokyo/Templates/Image|file=UT-Tokyo_Data-Top.png|caption=Figure 1. SMART ''E. coli''}} |
(A) Substrate-induced Cell Assembling System<br> | (A) Substrate-induced Cell Assembling System<br> | ||
| - | 1. Substrate (this time, an IPTG) induces expression from a substrate-induced promoter (BBa_R0011).<br> | + | 1. Substrate (this time, an IPTG) induces expression from a substrate-induced promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_R0011 BBa_R0011]).<br> |
| - | 2. ''aspA'' (BBa_C0083) is expressed.<br> | + | 2. ''aspA'' ([http://partsregistry.org/wiki/index.php?title=Part:BBa_C0083 BBa_C0083]) is expressed.<br> |
3. AspA catalyses the production of L-aspartate from fumarate and ammonium ion.<br> | 3. AspA catalyses the production of L-aspartate from fumarate and ammonium ion.<br> | ||
(B) Substrate-induced Cell Arrest System<br> | (B) Substrate-induced Cell Arrest System<br> | ||
cheZ<sup>-/-</sup> strain is cloned for the works below. <br> | cheZ<sup>-/-</sup> strain is cloned for the works below. <br> | ||
| - | 4. The | + | 4. The cI-repressed promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_R0051 BBa_R0051]) is active when the cI repressor is absent.<br> |
5. CheZ is expressed and rescues the motility of cheZ<sup>-/-</sup> strain.<br> | 5. CheZ is expressed and rescues the motility of cheZ<sup>-/-</sup> strain.<br> | ||
| - | 6. When | + | 6. When cI repressor ([http://partsregistry.org/wiki/index.php?title=Part:BBa_C0051 BBa_C0051]) is induced, it inhibits the activity of ''cI'' promotor. This results in the repression of CheZ expression, which leads to the loss of motility.<br> |
| + | |||
| - | == | + | ==Dual luciferase assay== |
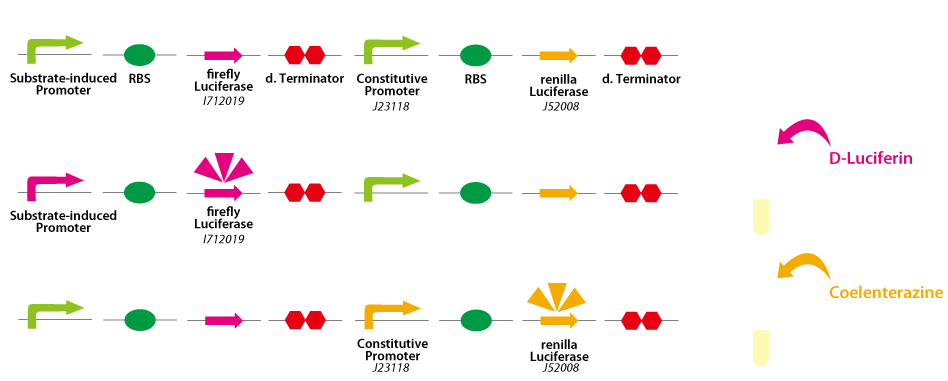
| - | 1. A constitutive promoter of known strength (this time, BBa_J23118) is placed upstream of renilla luciferase expression cassette (BBa_K518001), and a promoter of interest is placed upstream of firefly luciferase expression cassette (BBa_K518000).<br> | + | {{:Team:UT-Tokyo/Templates/Image|file=UT-Tokyo_Data_luciferase.png|caption=Figure 2. Dual luciferase assay}} |
| + | 1. A constitutive promoter of known strength (this time, [http://partsregistry.org/wiki/index.php?title=Part:BBa_J23118 BBa_J23118]) is placed upstream of renilla luciferase expression cassette ([http://partsregistry.org/wiki/index.php?title=Part:BBa_BBa_K518001 BBa_K518001]), and a promoter of interest is placed upstream of firefly luciferase expression cassette ([http://partsregistry.org/wiki/index.php?title=Part:BBa_BBa_K518000 BBa_K518000]).<br> | ||
2. After an instillation of first substrate D-luciferin, firefly luminescence is measured.<br> | 2. After an instillation of first substrate D-luciferin, firefly luminescence is measured.<br> | ||
| - | 3. After another substrate, coelenterazine, is added, renilla luminescence is measured as a reference to compute the ratio of the expression level of the target promoter to that of the internal control promoter (BBa_J23118).<br> | + | 3. After another substrate, coelenterazine, is added, renilla luminescence is measured as a reference to compute the ratio of the expression level of the target promoter to that of the internal control promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_J23118 BBa_J23118]).<br> |
| - | + | =Data for our favourite new parts= | |
| - | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K518007 BBa_K518007] '''cheZ expression cassette (no promoter)'''<br> | + | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K518007 BBa_K518007] '''''cheZ'' expression cassette (no promoter)'''<br> |
This construct rescues the mobility of cheZ<sup>-/-</sup> cells. | This construct rescues the mobility of cheZ<sup>-/-</sup> cells. | ||
| - | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K518010 BBa_K518010] '''sulA promoter'''<br> | + | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K518010 BBa_K518010] '''''sulA'' promoter'''<br> |
| - | This is a promoter of the sulA gene which is responsible for stress-induced arrest of cell division. | + | This is a promoter of the ''sulA'' gene which is responsible for stress-induced arrest of cell division. |
| - | We successfully demonstrated a significant alteration of expression of a gene downstream of this promoter after UV irradiation | + | We successfully demonstrated a significant alteration of expression of a gene downstream of this promoter after UV irradiation. This part can be utilized as a "signal induced promotor" in our system. |
| - | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K518013 BBa_K518013] '''sulA promoter evaluation device'''<br> | + | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K518013 BBa_K518013] '''''sulA'' promoter evaluation device'''<br> |
sulAp is known to respond to various types of DNA-injuring stress. | sulAp is known to respond to various types of DNA-injuring stress. | ||
This construct is a sulAp evaluation device to make it easy to compare the expression of sulAp in various "stressful" conditions. | This construct is a sulAp evaluation device to make it easy to compare the expression of sulAp in various "stressful" conditions. | ||
Employing our dual luciferase assay kit, both quantitative measurements and a comparison of Relative Promoter Unit (RPU) can be achieved. | Employing our dual luciferase assay kit, both quantitative measurements and a comparison of Relative Promoter Unit (RPU) can be achieved. | ||
| - | + | =Data for pre-existing parts= | |
[http://partsregistry.org/Part:BBa_I712019:Experience BBa_I712019] '''Firefly luciferase - luciferase from Photinus pyralis''' (Ljubljana, 2007)<br> | [http://partsregistry.org/Part:BBa_I712019:Experience BBa_I712019] '''Firefly luciferase - luciferase from Photinus pyralis''' (Ljubljana, 2007)<br> | ||
This is firefly luciferase which produces luminescence by oxidation of D-luciferin. <br> | This is firefly luciferase which produces luminescence by oxidation of D-luciferin. <br> | ||
| Line 43: | Line 45: | ||
We evaluated the relative expression levels of this promoter with various IPTG concentrations. | We evaluated the relative expression levels of this promoter with various IPTG concentrations. | ||
| - | + | =We’ve also characterized the following parts= | |
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518000 BBa_K518000] '''RBS + firefly luciferase + d.terminator'''<br> | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K518000 BBa_K518000] '''RBS + firefly luciferase + d.terminator'''<br> | ||
Firefly luciferase emits luminescence by the oxidation of D-luciferin. | Firefly luciferase emits luminescence by the oxidation of D-luciferin. | ||
| Line 68: | Line 70: | ||
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518008 BBa_K518008] '''IPTG-inducible CheZ repression device'''<br> | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K518008 BBa_K518008] '''IPTG-inducible CheZ repression device'''<br> | ||
| - | This construct usually allows strong | + | This construct usually allows strong CheZ expression, while in the presence of IPTG, the transcription of ''cheZ'' is repressed by the lambda ''cI'' repressor. |
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518012 BBa_K518012] '''RBS + RFP + d.Ter'''<br> | [http://partsregistry.org/wiki/index.php?title=Part:BBa_K518012 BBa_K518012] '''RBS + RFP + d.Ter'''<br> | ||
Latest revision as of 02:11, 6 October 2011
Data

iGEM UT-Tokyo
How our system works
SMART E. coli
(A) Substrate-induced Cell Assembling System
1. Substrate (this time, an IPTG) induces expression from a substrate-induced promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_R0011 BBa_R0011]).
2. aspA ([http://partsregistry.org/wiki/index.php?title=Part:BBa_C0083 BBa_C0083]) is expressed.
3. AspA catalyses the production of L-aspartate from fumarate and ammonium ion.
(B) Substrate-induced Cell Arrest System
cheZ-/- strain is cloned for the works below.
4. The cI-repressed promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_R0051 BBa_R0051]) is active when the cI repressor is absent.
5. CheZ is expressed and rescues the motility of cheZ-/- strain.
6. When cI repressor ([http://partsregistry.org/wiki/index.php?title=Part:BBa_C0051 BBa_C0051]) is induced, it inhibits the activity of cI promotor. This results in the repression of CheZ expression, which leads to the loss of motility.
Dual luciferase assay
1. A constitutive promoter of known strength (this time, [http://partsregistry.org/wiki/index.php?title=Part:BBa_J23118 BBa_J23118]) is placed upstream of renilla luciferase expression cassette ([http://partsregistry.org/wiki/index.php?title=Part:BBa_BBa_K518001 BBa_K518001]), and a promoter of interest is placed upstream of firefly luciferase expression cassette ([http://partsregistry.org/wiki/index.php?title=Part:BBa_BBa_K518000 BBa_K518000]).
2. After an instillation of first substrate D-luciferin, firefly luminescence is measured.
3. After another substrate, coelenterazine, is added, renilla luminescence is measured as a reference to compute the ratio of the expression level of the target promoter to that of the internal control promoter ([http://partsregistry.org/wiki/index.php?title=Part:BBa_J23118 BBa_J23118]).
Data for our favourite new parts
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518007 BBa_K518007] cheZ expression cassette (no promoter)
This construct rescues the mobility of cheZ-/- cells.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518010 BBa_K518010] sulA promoter
This is a promoter of the sulA gene which is responsible for stress-induced arrest of cell division.
We successfully demonstrated a significant alteration of expression of a gene downstream of this promoter after UV irradiation. This part can be utilized as a "signal induced promotor" in our system.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518013 BBa_K518013] sulA promoter evaluation device
sulAp is known to respond to various types of DNA-injuring stress.
This construct is a sulAp evaluation device to make it easy to compare the expression of sulAp in various "stressful" conditions.
Employing our dual luciferase assay kit, both quantitative measurements and a comparison of Relative Promoter Unit (RPU) can be achieved.
Data for pre-existing parts
[http://partsregistry.org/Part:BBa_I712019:Experience BBa_I712019] Firefly luciferase - luciferase from Photinus pyralis (Ljubljana, 2007)
This is firefly luciferase which produces luminescence by oxidation of D-luciferin.
We utilized this part to devise a Firefly-Renilla Dual Luciferase Assay Kit.
[http://partsregistry.org/Part:BBa_J52008:Experience BBa_J52008] luciferase: luciferin 2-monooxygenase from Renilla reniformis (Slovenia, 2006)
This is Renilla luciferase which emits luminescence when coelenterazine is added.
We utilized this part to devise a Firefly-Renilla Dual Luciferase Assay Kit.
[http://partsregistry.org/Part:BBa_R0011:Experience BBa_R0011] Promoter (lacI regulated, lambda pL hybrid) (Neelaksh Varshney et al., 2003)
We evaluated the relative expression levels of this promoter with various IPTG concentrations.
We’ve also characterized the following parts
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518000 BBa_K518000] RBS + firefly luciferase + d.terminator
Firefly luciferase emits luminescence by the oxidation of D-luciferin.
This construct can be used as a measuring tool when combined with other cis-elements, because of its high quantitative performance.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518001 BBa_K518001] RBS + renilla luciferase + d.terminator
Renilla luciferase emits luminescence by the oxidation of coelenterazine.
This construct can be used as a measuring tool when combined with other cis-elements, because of its high quantitative performance.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518002 BBa_K518002] Firefly-renilla dual luciferase assay kit
This construct enables Luciferase assay which is one of the most popular reporter assay system for quantitatively measuring the strength of promoters and other cis-elements.
The wide-range and quantitative detection are the prominent features of this assay.
A promoter or other cis-elements to be analysed can be ligated upstream of this part.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518004 BBa_K518004] IPTG-inducible L-Asp producing device
This construct enables production of aspartate in the presence of enough substrate (fumaric acid and NH4+).
IPTG can be used to induce aspartate production.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518005 BBa_K518005] cheZ
CheZ is responsible for the dephosphorylation of the flagellum-regulating protein CheY.
Non-phosphorylated CheY results in E. coli swimming straight.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518006 BBa_K518006] IPTG-inducible CheZ expression device
This construct rescues cheZ-/- cell motility in the presence of IPTG.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518008 BBa_K518008] IPTG-inducible CheZ repression device
This construct usually allows strong CheZ expression, while in the presence of IPTG, the transcription of cheZ is repressed by the lambda cI repressor.
[http://partsregistry.org/wiki/index.php?title=Part:BBa_K518012 BBa_K518012] RBS + RFP + d.Ter
This part is an easy promoter assessment tool. The RFP reporter aids you to find out whether a promoter of your interest works.
 "
"