Team:Washington/Celiacs/Future
From 2011.igem.org
(→Kinetic Characterization) |
|||
| (13 intermediate revisions not shown) | |||
| Line 6: | Line 6: | ||
====Kinetic Characterization==== | ====Kinetic Characterization==== | ||
| - | We intend to develop a mass spectroscopy assay to measure the | + | We intend to develop a mass spectroscopy assay to measure the ''k<sub>cat</sub>'' (maximum rate of substrate turnover per molecule of enzyme per unit of time) and ''K<sub>M</sub>'' (Michaelis-Menten binding constant) values for our best mutant. |
| - | + | ||
| - | + | ||
====Crystal Structure==== | ====Crystal Structure==== | ||
| - | Protein structures can be obtained by crystallizing the protein, then bombarding it with X-rays and analyzing the diffraction pattern. Several crystal structures for wild-type Kumamolisin-As have already been published. To obtain further structural information, we hope to eventually obtain a crystal structure of our best mutated enzyme ( | + | Protein structures can be obtained by crystallizing the protein, then bombarding it with X-rays and analyzing the diffraction pattern. Several crystal structures for wild-type Kumamolisin-As have already been published. To obtain further structural information, we hope to eventually obtain a crystal structure of our best mutated enzyme (KumaMax) bound to the PQLP model substrate. This will involve mutating one of the residues in the catalytic triad, so that the substrate will remain bound without being cleaved. The structural information that we may be able to glean from such a structure will allow us to better characterize, and perhaps further improve our mutant. |
====Biophysical Characterization==== | ====Biophysical Characterization==== | ||
| Line 18: | Line 16: | ||
Once the ideal mutations are isolated, we intend to test the best mutants at gastric pH and in the presence of other gastric enzymes for a short period of time, mimicking the environment after enzyme ingestion and prior to enzyme uptake by the small intestine. We suspect that the thermostable properties of Kumamolisin-As will render our mutant enzyme reasonably resistant to degradation by gastric enzymes such as pepsin. However, if this proves not to be the case, we intend to reengineer the mutant for enhanced resistance to pepsin and other such gastric enzymes. Once ensured that the mutated Kumamolisin-As remains active under stomach conditions, this ideal mutated enzyme will be ready for ''in vivo'' experimentation. | Once the ideal mutations are isolated, we intend to test the best mutants at gastric pH and in the presence of other gastric enzymes for a short period of time, mimicking the environment after enzyme ingestion and prior to enzyme uptake by the small intestine. We suspect that the thermostable properties of Kumamolisin-As will render our mutant enzyme reasonably resistant to degradation by gastric enzymes such as pepsin. However, if this proves not to be the case, we intend to reengineer the mutant for enhanced resistance to pepsin and other such gastric enzymes. Once ensured that the mutated Kumamolisin-As remains active under stomach conditions, this ideal mutated enzyme will be ready for ''in vivo'' experimentation. | ||
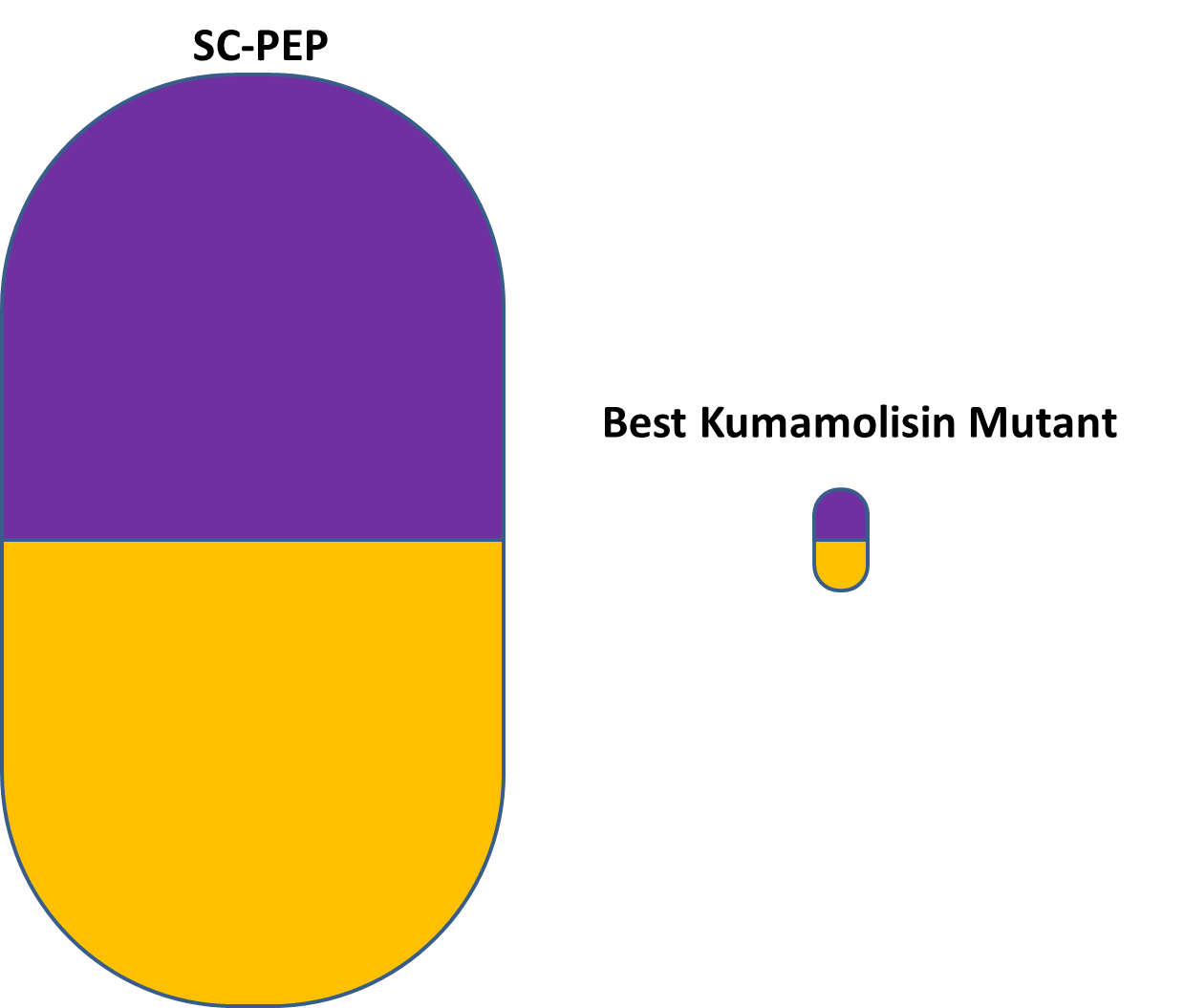
| - | [[File: | + | [[File:Washington_Kumamolisin_VS_SC-PEP.png|250px|thumb|right|Relative size of pills containing SC-PEP and our best Kumamolisin-As mutant (KumaMax), to achieve the same effect.]] |
| - | + | ||
| - | + | ||
====Therapeutic Promise==== | ====Therapeutic Promise==== | ||
| - | + | If, after successful completion of ''in vivo'' experimentation, our best mutated enzyme appears suited for pharmacological use, it could potentially lead to a treatment of gluten intolerance to be taken in pill form, along with gluten-containing meals. In other words, this engineered enzyme could someday allow people suffering from gluten intolerance to enjoy foods like pasta, pizza, and fresh bread. Due to the thermostable properties of Kumamolisin-As, our Kumamolisin-As mutant enzyme therapeutic could most likely be stored at room temperature. Moreover, since our Kumamolisin-As mutant is over 700 times as active as SC-PEP at the low pH of the stomach, a much smaller amount of it would be needed to achieve the same effect - allowing for pills that are both smaller and more cost effective to produce than a pill relying on SC-PEP would need to be. | |
Latest revision as of 06:58, 13 October 2011
Kinetic Characterization
We intend to develop a mass spectroscopy assay to measure the kcat (maximum rate of substrate turnover per molecule of enzyme per unit of time) and KM (Michaelis-Menten binding constant) values for our best mutant.
Crystal Structure
Protein structures can be obtained by crystallizing the protein, then bombarding it with X-rays and analyzing the diffraction pattern. Several crystal structures for wild-type Kumamolisin-As have already been published. To obtain further structural information, we hope to eventually obtain a crystal structure of our best mutated enzyme (KumaMax) bound to the PQLP model substrate. This will involve mutating one of the residues in the catalytic triad, so that the substrate will remain bound without being cleaved. The structural information that we may be able to glean from such a structure will allow us to better characterize, and perhaps further improve our mutant.
Biophysical Characterization
Once the ideal mutations are isolated, we intend to test the best mutants at gastric pH and in the presence of other gastric enzymes for a short period of time, mimicking the environment after enzyme ingestion and prior to enzyme uptake by the small intestine. We suspect that the thermostable properties of Kumamolisin-As will render our mutant enzyme reasonably resistant to degradation by gastric enzymes such as pepsin. However, if this proves not to be the case, we intend to reengineer the mutant for enhanced resistance to pepsin and other such gastric enzymes. Once ensured that the mutated Kumamolisin-As remains active under stomach conditions, this ideal mutated enzyme will be ready for in vivo experimentation.
Therapeutic Promise
If, after successful completion of in vivo experimentation, our best mutated enzyme appears suited for pharmacological use, it could potentially lead to a treatment of gluten intolerance to be taken in pill form, along with gluten-containing meals. In other words, this engineered enzyme could someday allow people suffering from gluten intolerance to enjoy foods like pasta, pizza, and fresh bread. Due to the thermostable properties of Kumamolisin-As, our Kumamolisin-As mutant enzyme therapeutic could most likely be stored at room temperature. Moreover, since our Kumamolisin-As mutant is over 700 times as active as SC-PEP at the low pH of the stomach, a much smaller amount of it would be needed to achieve the same effect - allowing for pills that are both smaller and more cost effective to produce than a pill relying on SC-PEP would need to be.
 "
"