Team:Paris Bettencourt/GFP diff
From 2011.igem.org
| (60 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:Paris_Bettencourt/tpl_test}} | {{:Team:Paris_Bettencourt/tpl_test}} | ||
<html> | <html> | ||
| + | <h1>GFP diffusion</h1> | ||
| + | <h2>Design of the experiment</h2> | ||
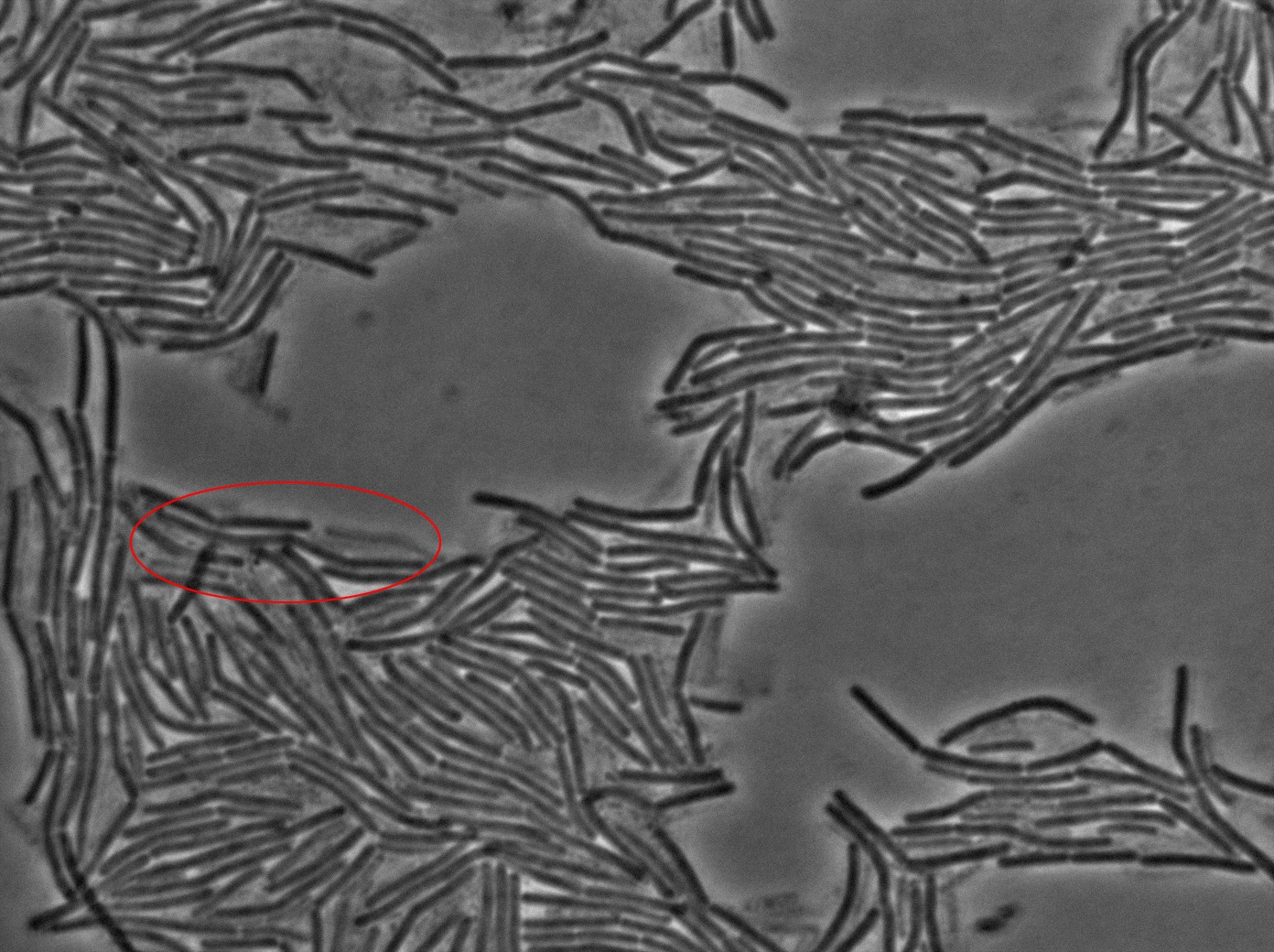
| + | <p>The key experiment of the Dubey and Ben-Yehuda paper <a href="https://2011.igem.org/Team:Paris_Bettencourt/GFP_diff#references">[1]</a> is simple. They used one strain of <i>gfp + B.subtilis</i> and one <i>gfp - B.subtilis</i> strain. The two strains of <i>B.subtilis</i> in exponential phase were mixed (ratio 1:1) on an LB-agarose (1.5%) slab and sealed in a microscope slide cavity. The aim is to obtain a <em>monolayer of densely packed cells</em>.</p> | ||
| + | <center><img src="https://static.igem.org/mediawiki/2011/b/b4/Gfp_diff.png" style="width:800px"></center> | ||
| + | <center>GFP diffusion through the nanotubes</center> | ||
| + | <p>The plated bacteria were then observed by time-lapse fluorescent microscopy. After a while (between 15 minutes and 2 hours), a transfer of GFP can be observed from the <i>gfp +</i> cells towards the <i>gfp-</i> cells. This cell-to-cell communication was previously unheard of and the original paper <a href="http://bms.ucsf.edu/sites/ucsf-bms.ixm.ca/files/marjordan_06022011.pdf">[1]</a> strongly suggest that the nanotubes observed through electronic microscopy by the authors are the reason of this transfer.</p> | ||
| + | <h2>Our results</h2> | ||
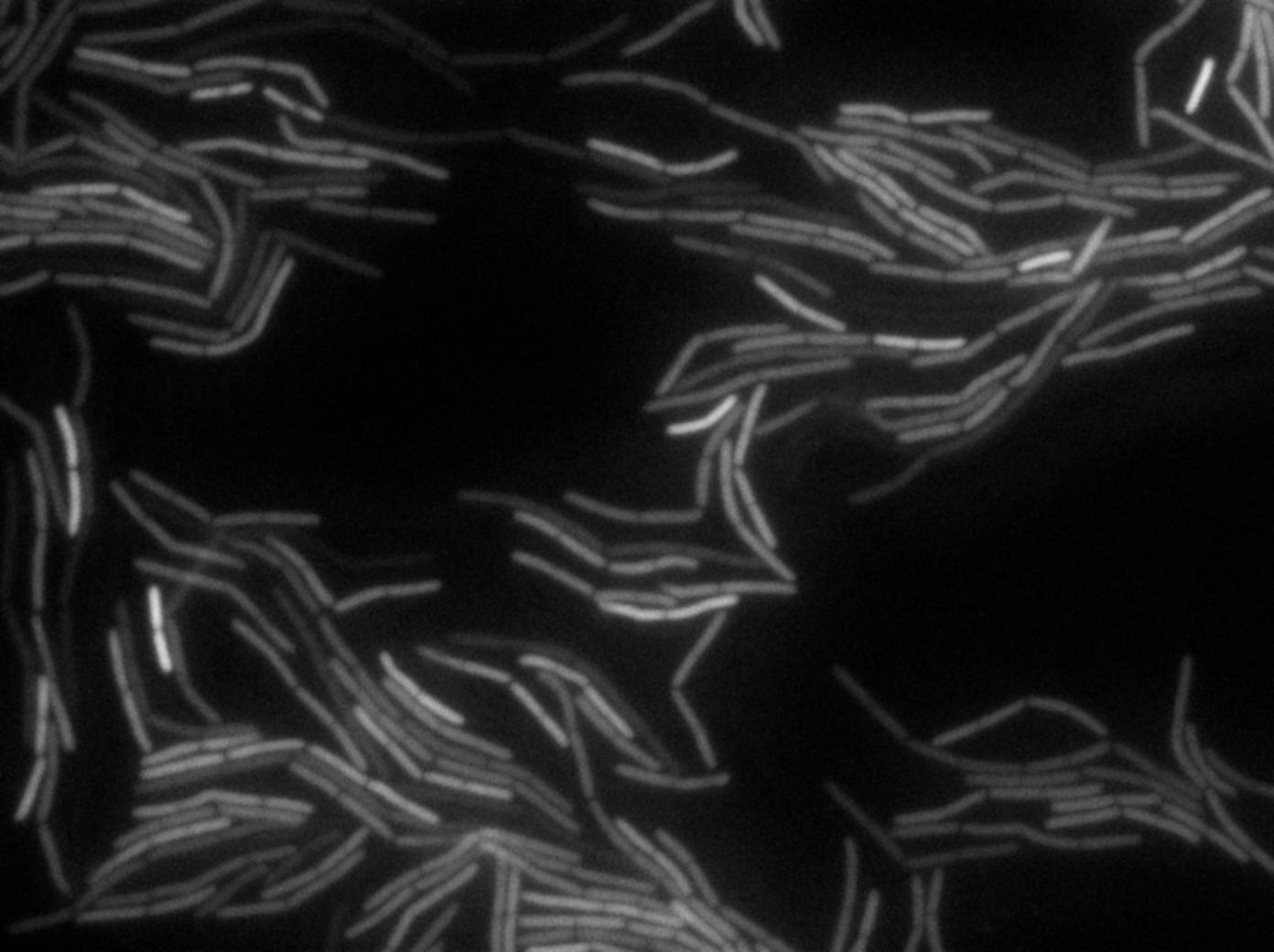
| + | <p>We began our experiments with PY79 <i>B.subtilis</i> strain (as in the original paper) but observed that the fluorescence of this strain was weak. We then chose to work with a 3610 strain which showed stronger fluorescence .</p> | ||
| + | <p>We <em>reproduced the result expected: GFP diffusion between cells!</em></p> | ||
| + | <center><img src="https://static.igem.org/mediawiki/2011/1/1e/Fluofordummies.png" style="width:800px"></center> | ||
| - | == GFP | + | <br> |
| - | + | <p>You can find out more about this exciting day in the <a href="https://2011.igem.org/Team:Paris_Liliane_Bettencourt/Notebook/2011/09/15/">Notebook</a>.</p> | |
| - | + | <p>Regarding the microscopy protocol you can refer to this <a href="https://2011.igem.org/Team:Paris_Bettencourt/Experiments/Microscopy">page</a>.</p> | |
| - | + | <p>You can find the video of our results on this <a href="http://www.youtube.com/watch?v=OwURHcJYPBo">link</a>.</p> | |
| - | + | <center><iframe width="420" height="315" src="http://www.youtube.com/embed/OwURHcJYPBo" frameborder="0" allowfullscreen></iframe></center> | |
| + | <p>The important time steps of this experiment can be found below:</p> | ||
| + | </html> | ||
| + | {| border="1" class="wikitable" style="text-align: center;" | ||
| + | |+3610 gfp-/3610 gfp+ in exponential phase at 37°C | ||
| + | |- | ||
| + | |[[File:0914_3610_classic_w1TRANS_s5_t1.jpg|450px|thumb|center|3610 gfp- and 3610 gfp+ strains at 37°C t=0 min position 5 (trans image)]] | ||
| + | |[[File:gfp_diff_fluo_0001.jpg|450px|thumb|center|3610 gfp- and 3610 gfp+ strains at 37°C t=0 min position 5 (gfp image)]] | ||
| + | |- | ||
| + | |[[File:0914_3610_classic_w1TRANS_s5_t9.jpg|450px|thumb|center|3610 gfp- and 3610 gfp+ strains at 37°C t=45 min position 5 (trans image)]] | ||
| + | |[[File:gfp_diff_fluo_0002.jpg|450px|thumb|center|3610 gfp- and 3610 gfp+ strains at 37°C t=45 min position 5 (gfp image)]] | ||
| + | |- | ||
| + | |[[File:0914_3610_classic_w1TRANS_s5_t12.jpg|450px|thumb|center|3610 gfp- and 3610 gfp+ strains at 37°C t=60 min position 5 (trans image)]] | ||
| + | |[[File:gfp_diff_fluo_0003.jpg|450px|thumb|center|3610 gfp- and 3610 gfp+ at 37°C t=60 min position 5 (gfp image)]] | ||
| + | |- | ||
| + | |[[File:0914_3610_classic_w1TRANS_s5_t23.png|450px|thumb|center|3610 gfp- and 3610 gfp+ strains at 37°C t=180 min position 5 (trans image)]] | ||
| + | |[[File:gfp_diff_fluo_0004.jpg|450px|thumb|center|3610 gfp- and 3610 gfp+ strains at 37°C t=180 min position 5 (gfp image)]] | ||
| + | |} | ||
| + | <html> | ||
| + | <h2>Conclusions of our experiment</h2> | ||
| + | <ul> | ||
| + | <li>We have <em>evidence of a cell-to-cell GFP transfer</em>. This is probably a non-specific transfer (as GFP is not a natural protein for <i>B.subtilis</i>) and according to the Dubey and Ben-Yehuda paper <a href="http://bms.ucsf.edu/sites/ucsf-bms.ixm.ca/files/marjordan_06022011.pdf">[1]</a>, we can strongly suspect that the GFP transfer is through nanotubes.</li> | ||
| + | <li>Reproducing the original experiment is difficult. We were able to observe the expected behaviour in <em>very few cells</em>. This means either that the transfer is very rare or that we need to improve the preparation of the slide.</li> | ||
| + | <li>We used a different strain (3610 instead of PY79). This means the <em>transfer is not observed only in PY79</em>.</li> | ||
| + | <li>We proved that we will be able to see the results of our designs once we test them.</li> | ||
| + | <ul> | ||
| + | <br> | ||
| + | <div id="citation_box"> | ||
| + | <p id="references">References</p> | ||
| + | <ol> | ||
| + | <li><i>Intercellular Nanotubes Mediate Bacterial Communication</i>, Dubey and Ben-Yehuda, Cell, available <a href="http://bms.ucsf.edu/sites/ucsf-bms.ixm.ca/files/marjordan_06022011.pdf">here</a></li> | ||
| + | </ol> | ||
| + | </div> | ||
| + | <br> | ||
<!-- PAGE FOOTER -- ITEMS FROM COLUMN ! HAVE BEEN MOVED HERE -- RDR --> | <!-- PAGE FOOTER -- ITEMS FROM COLUMN ! HAVE BEEN MOVED HERE -- RDR --> | ||
| Line 60: | Line 103: | ||
</div> | </div> | ||
</div> | </div> | ||
| + | </div> | ||
| + | |||
| + | <div id="scroll_right"><a href="https://2011.igem.org/Team:Paris_Bettencourt/Experiments/Microscopy"><img src="https://static.igem.org/mediawiki/2011/e/e0/Arrow-right-big.png" style="width:100%;"></a><a href="https://2011.igem.org/Team:Paris_Bettencourt/Experiments/Microscopy">Microscopy protocol</a></div> | ||
</body> | </body> | ||
</html> | </html> | ||
Latest revision as of 19:35, 18 October 2011

GFP diffusion
Design of the experiment
The key experiment of the Dubey and Ben-Yehuda paper [1] is simple. They used one strain of gfp + B.subtilis and one gfp - B.subtilis strain. The two strains of B.subtilis in exponential phase were mixed (ratio 1:1) on an LB-agarose (1.5%) slab and sealed in a microscope slide cavity. The aim is to obtain a monolayer of densely packed cells.

The plated bacteria were then observed by time-lapse fluorescent microscopy. After a while (between 15 minutes and 2 hours), a transfer of GFP can be observed from the gfp + cells towards the gfp- cells. This cell-to-cell communication was previously unheard of and the original paper [1] strongly suggest that the nanotubes observed through electronic microscopy by the authors are the reason of this transfer.
Our results
We began our experiments with PY79 B.subtilis strain (as in the original paper) but observed that the fluorescence of this strain was weak. We then chose to work with a 3610 strain which showed stronger fluorescence .
We reproduced the result expected: GFP diffusion between cells!

You can find out more about this exciting day in the Notebook.
Regarding the microscopy protocol you can refer to this page.
You can find the video of our results on this link.
The important time steps of this experiment can be found below:
Conclusions of our experiment
- We have evidence of a cell-to-cell GFP transfer. This is probably a non-specific transfer (as GFP is not a natural protein for B.subtilis) and according to the Dubey and Ben-Yehuda paper [1], we can strongly suspect that the GFP transfer is through nanotubes.
- Reproducing the original experiment is difficult. We were able to observe the expected behaviour in very few cells. This means either that the transfer is very rare or that we need to improve the preparation of the slide.
- We used a different strain (3610 instead of PY79). This means the transfer is not observed only in PY79.
- We proved that we will be able to see the results of our designs once we test them.
- Intercellular Nanotubes Mediate Bacterial Communication, Dubey and Ben-Yehuda, Cell, available here
References
 "
"