Team:EPF-Lausanne/Protocols/MG Agar Plates
From 2011.igem.org
(Created page with "{{:Team:EPF-Lausanne/Templates/ProtocolHeader|title=Agar plate preparation}} The Agar plates are prepared in the same way as [[Team:EPF-Lausanne/Protocols/Agar Plates|those for ...") |
(→Agar broth) |
||
| (One intermediate revision not shown) | |||
| Line 26: | Line 26: | ||
* Keep in 55° C bath after autoclaving (that's where it will arrive if autoclaved in Bart's lab). | * Keep in 55° C bath after autoclaving (that's where it will arrive if autoclaved in Bart's lab). | ||
* Mix on heating mixer, until homogeneous (don't close lid completely). | * Mix on heating mixer, until homogeneous (don't close lid completely). | ||
| + | * Once autoclaved and homogeneous, add: | ||
| + | |||
| + | {| | ||
| + | |+ To add after autoclaving | ||
| + | ! Product || Amount | ||
| + | |- | ||
| + | | 1M CaCl2 || 1 ml | ||
| + | |- | ||
| + | | 5 mg/ml Cholesterol in Ethanol || 1 ml | ||
| + | |- | ||
| + | | 1M MgSO4 || 1ml | ||
| + | |- | ||
| + | | 1M KPO4 buffer || 25 ml | ||
| + | |} | ||
| Line 31: | Line 45: | ||
* the broth starts gelification as soon as it cools down. Store it in a warm bath to keep it liquid. If it does gelify, just warm and mix again (with magnetic mixer). | * the broth starts gelification as soon as it cools down. Store it in a warm bath to keep it liquid. If it does gelify, just warm and mix again (with magnetic mixer). | ||
* always work around a Bunsen burner flame, to avoid contamination. | * always work around a Bunsen burner flame, to avoid contamination. | ||
| - | |||
== Pouring the plates == | == Pouring the plates == | ||
Latest revision as of 12:15, 2 September 2011
Agar plate preparation
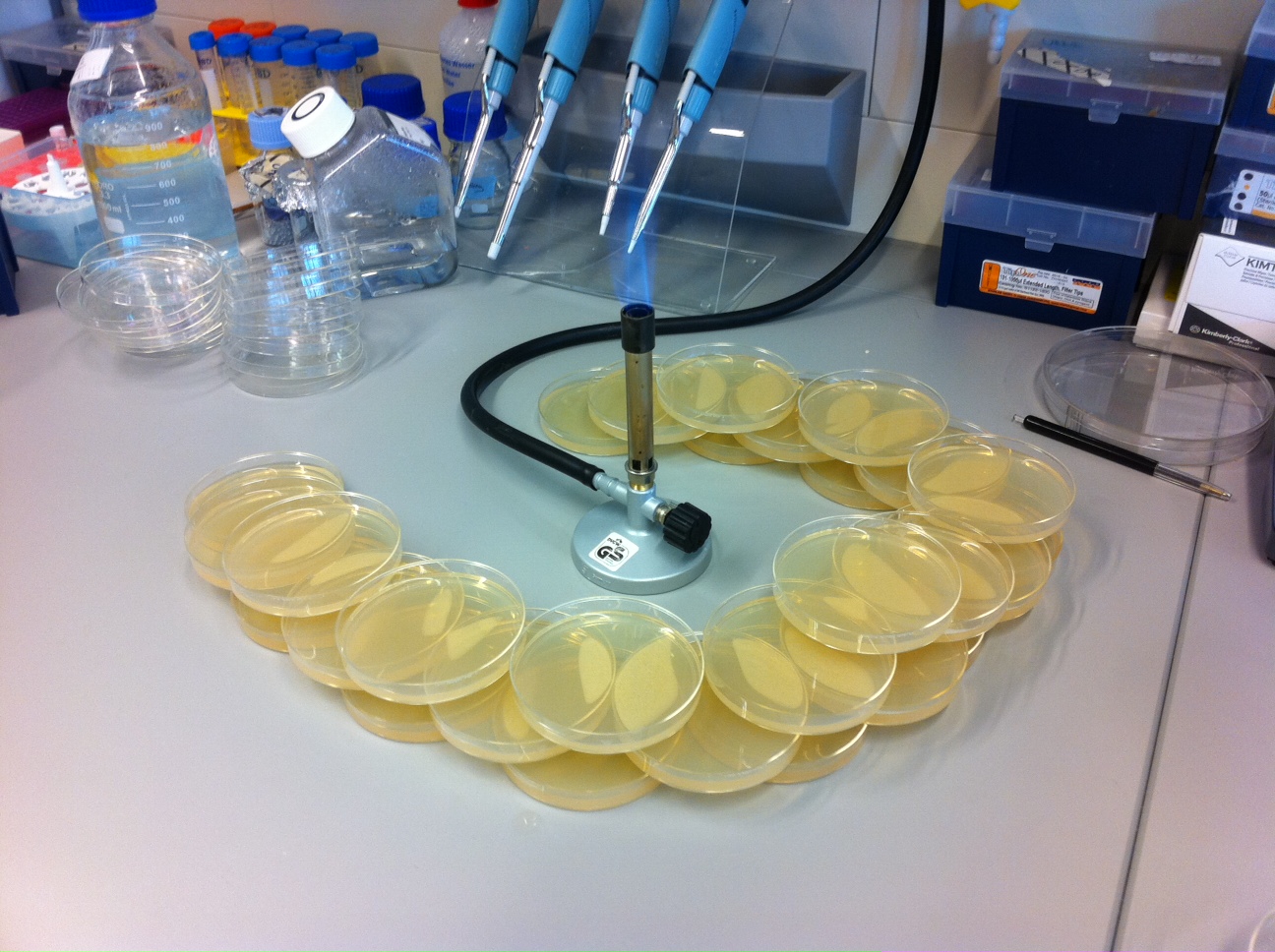
Back to protocols.The Agar plates are prepared in the same way as those for cell cultures. First an agar broth is mixed and autoclaved. The solution is poured into disposable petri plates and let gelify for 20 minutes around a bunzen burner flame, as illustrated below. Finally, the plates are left at room temperature, covered, for two to three days, to detect for traces of contamination.
Agar broth
Mix the following in a large bottle:
| Product | Amount |
|---|---|
| NaCl | 3 g |
| Peptone | 2.5 g |
| Agar | 17 g |
| H2O (deionised) | fill to 1 l |
- Autoclave.
- Keep in 55° C bath after autoclaving (that's where it will arrive if autoclaved in Bart's lab).
- Mix on heating mixer, until homogeneous (don't close lid completely).
- Once autoclaved and homogeneous, add:
| Product | Amount |
|---|---|
| 1M CaCl2 | 1 ml |
| 5 mg/ml Cholesterol in Ethanol | 1 ml |
| 1M MgSO4 | 1ml |
| 1M KPO4 buffer | 25 ml |
Warnings:
- the broth starts gelification as soon as it cools down. Store it in a warm bath to keep it liquid. If it does gelify, just warm and mix again (with magnetic mixer).
- always work around a Bunsen burner flame, to avoid contamination.
Pouring the plates
Arrange empty petri dishes in a crescent around a Bunzen burner. Half-fill them with agar broth. Stack another layer of empty dishes above, and repeat until all the broth has been poured. One liter of broth fills approximately 30 plates.
Leave the plates around the flame for approximately twenty minutes, until the gel is solidified. Then replace lid and store in fridge. Leave them out for two to three days, and discard any plates that show signs of contamination.
References
- Personnal protocols of Lukas Von Tobel, from the [http://gonczy-lab.epfl.ch/ Gönczy Lab].
- Stiernagle, T. Maintenance of C. elegans (February 11, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.101.1, http://www.wormbook.org.
 "
"