Team:HKU-Hong Kong/Lab Protocol
From 2011.igem.org
(Difference between revisions)
| (53 intermediate revisions not shown) | |||
| Line 71: | Line 71: | ||
|style="width:900px;| | |style="width:900px;| | ||
<OL> | <OL> | ||
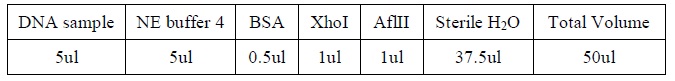
| - | <LI>Add the following reagents, with the enzymes added at the last, into a tube.</ | + | <LI>Add the following reagents, with the enzymes added at the last, into a tube |
| - | <LI>All steps should be carried out on ice. | + | <div ALIGN=CENTER> |
| - | <LI>Mix well after addition of all the reagent. | + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; |
| - | <LI>Incubate the mixture at 37oC for several hours. | + | |- |
| - | + | |[[Image:Table_1.jpg|500px]] | |
| + | |- | ||
| + | |Reagents for DNA digestion. | ||
| + | |} | ||
| + | </div> | ||
| + | <LI>All steps should be carried out on ice. | ||
| + | <LI>Mix well after addition of all the reagent. | ||
| + | <LI>Incubate the mixture at 37oC for several hours. | ||
|- | |- | ||
| Line 104: | Line 111: | ||
|style="width:900px;| | |style="width:900px;| | ||
<OL> | <OL> | ||
| - | Colony PCR | + | <LI>Colony PCR |
<OL> | <OL> | ||
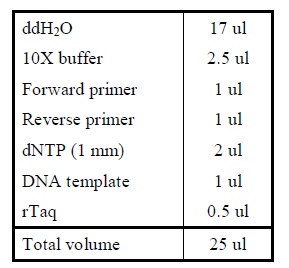
| - | <LI>Add the following reagents into a PCR tube (in order) and mix well.</ | + | <LI>Add the following reagents into a PCR tube (in order) and mix well. |
| - | <LI>Set the following PCR program.</ | + | <div ALIGN=CENTER> |
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Colony_PCR.jpg|225px]] | ||
| + | |- | ||
| + | |Reagents for colony PCR. | ||
| + | |} | ||
| + | </div> | ||
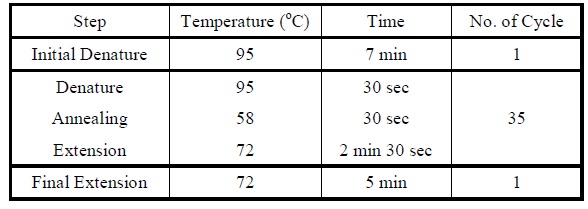
| + | <LI>Set the following PCR program. | ||
| + | <div ALIGN=CENTER> | ||
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Colony_pcr_program.jpg|475px]] | ||
| + | |- | ||
| + | |Reaction program for colony PCR. | ||
| + | |} | ||
| + | </div> | ||
</OL> | </OL> | ||
| - | Reverse PCR | + | <LI>Reverse PCR |
<OL> | <OL> | ||
| - | <LI>Add the following reagents into a PCR tube (in order) and mix well.</ | + | <LI>Add the following reagents into a PCR tube (in order) and mix well. |
| - | <LI>Set the following PCR program.</ | + | <div ALIGN=CENTER> |
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Reverse_pcr.jpg|225px]] | ||
| + | |- | ||
| + | |Reagents for reverse PCR. | ||
| + | |} | ||
| + | </div> | ||
| + | <LI>Set the following PCR program. | ||
| + | <div ALIGN=CENTER> | ||
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Reverse_pcr_program.jpg|475px]] | ||
| + | |- | ||
| + | |Reaction program for reverse PCR. | ||
| + | |} | ||
| + | </div> | ||
</OL> | </OL> | ||
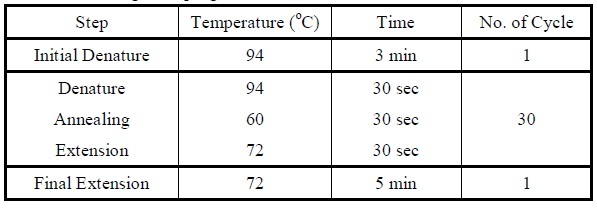
| - | Overlap PCR | + | <LI>Overlap PCR |
<OL> | <OL> | ||
| - | <LI>First, two PCR reactions are set for amplifying the two genes, tetR and HNS, separately. | + | <LI>First, two PCR reactions are set for amplifying the two genes, tetR and HNS, separately. |
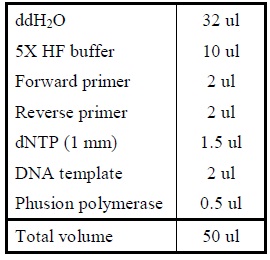
| - | <LI>Add the following reagents into a PCR tube (in order) and mix well.</ | + | <LI>Add the following reagents into a PCR tube (in order) and mix well. |
| - | <LI>Set the following PCR program.</ | + | <div ALIGN=CENTER> |
| - | <LI>Set up another PCR reaction using a primer with a linker to link the two genes. | + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; |
| - | <LI>Add the following reagents into a PCR tube (in order) and mix well.</ | + | |- |
| - | <LI>Set the following PCR program.</ | + | |[[Image:Overlap_pcr.jpg|500px]] |
| - | <LI>Set up another PCR reaction to further amplify the fused product. | + | |- |
| - | <LI>Add the following reagents into a PCR tube (in order) and mix well.</ | + | |Reagents for amplifying genes. |
| - | <LI>Set the following PCR program. | + | |} |
| - | < | + | </div> |
| - | </ | + | <LI>Set the following PCR program. |
| + | <div ALIGN=CENTER> | ||
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Overlap_pcr_program.jpg|500px]] | ||
| + | |- | ||
| + | |Reaction program for amplifying genes. | ||
| + | |} | ||
| + | </div> | ||
| + | <LI>Set up another PCR reaction using a primer with a linker to link the two genes. | ||
| + | <LI>Add the following reagents into a PCR tube (in order) and mix well. | ||
| + | <div ALIGN=CENTER> | ||
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Overlap_pcr_program.jpg|500px]] | ||
| + | |- | ||
| + | |Reagents for linking two genes. | ||
| + | |} | ||
| + | </div> | ||
| + | <LI>Set the following PCR program. | ||
| + | <div ALIGN=CENTER> | ||
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Overlap_pcr_program_2.jpg|500px]] | ||
| + | |- | ||
| + | |Reaction program for linking two genes. | ||
| + | |} | ||
| + | </div> | ||
| + | <LI>Set up another PCR reaction to further amplify the fused product. | ||
| + | <LI>Add the following reagents into a PCR tube (in order) and mix well. | ||
| + | <div ALIGN=CENTER> | ||
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Overlap_pcr_3.jpg|300px]] | ||
| + | |- | ||
| + | |Reagents for amplifying the fused product. | ||
| + | |} | ||
| + | </div> | ||
| + | <LI>Set the following PCR program. | ||
| + | <div ALIGN=CENTER> | ||
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Overlap_pcr_program_3.jpg|500px]] | ||
| + | |- | ||
| + | |Reaction program for amplifying the fused product. | ||
| + | |} | ||
| + | </div> | ||
| + | |||
|- | |- | ||
| Line 135: | Line 221: | ||
|style="width:900px;| | |style="width:900px;| | ||
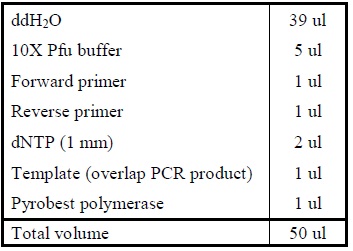
<OL> | <OL> | ||
| - | <LI>Add the following reagents, with the enzymes added at the last, into a tube.</ | + | <LI>Add the following reagents, with the enzymes added at the last, into a tube. |
| - | <LI>Incubate at 16oC overnight. | + | <div ALIGN=CENTER> |
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:DNA_ligation.jpg|550px]] | ||
| + | |- | ||
| + | |Composition for DNA ligation. | ||
| + | |} | ||
| + | </div> | ||
| + | <LI>Incubate at 16oC overnight. | ||
</OL> | </OL> | ||
| Line 150: | Line 244: | ||
|style="width:900px;font-size: 1.7em"|'''B. BACTERIAL WORK''' | |style="width:900px;font-size: 1.7em"|'''B. BACTERIAL WORK''' | ||
|- | |- | ||
| + | |style="width:900px;font-size: 1.3em;"|Overnight culture | ||
| + | |- | ||
| + | |style="width:900px;| | ||
| + | <OL> | ||
| + | <LI>Pipette 3 mL of LB broth into a culture tube.</LI> | ||
| + | <LI>Add 3 ul of Ampicillin or 3 ul of Chloramphenicol.</LI> | ||
| + | <LI>Pick a single colony by a sterile pipette tip.</LI> | ||
| + | <LI>Place the culture tube in the rotary shaker and incubate at 37oC overnight.</LI> | ||
| + | </OL> | ||
| + | |||
| + | |- | ||
| + | |style="width:900px;font-size: 1.3em;"|Preparation of competent cell | ||
| + | |- | ||
| + | |style="width:900px;| | ||
| + | <OL> | ||
| + | <LI>Seed culture:</LI> | ||
| + | <OL> | ||
| + | <LI>Pick a single colony from a plate with fresh grown cells (for 16 – 20 hours at 37oC) and transfer it into 3 mL of LB broth in a sterilized 15-mL polypropylene tube.</LI> | ||
| + | <LI>Incubate the culture overnight at 37oC in a rotatory shaker to provide vigorous shaking.</LI> | ||
| + | </OL> | ||
| + | <LI>Main culture:</LI> | ||
| + | <OL> | ||
| + | <LI>Inoculate 1,000 ul of seed culture into 100 mL of LB broth in a sterile 250-mL flask.</LI> | ||
| + | <LI>Incubate the culture at 37oC with vigorous shaking (in a rotary shaker) for approximately 2 hours or until the OD600 value reaches 0.3 to 0.4.</LI> | ||
| + | </OL> | ||
| + | <LI>Aseptically transfer the cells to a sterilized, chilled 50-mL polypropylene tube and cool the cultures to 0oC by placing the tube on ice for 10 minutes.</LI> | ||
| + | <LI>Centrifuge at 4,000 rpm for 5 – 15 minutes at 4oC.</LI> | ||
| + | <LI>Decant the media from the cell pellets.</LI> | ||
| + | <LI>Resuspend the cell pellets in 20 mL of filtered, sterilized, chilled 0.1M calcium chloride (CaCl2).</LI> | ||
| + | <LI>Vortex gently to mix it and place the tube on ice for 15 to 30 minutes.</LI> | ||
| + | <LI>Centrifuge at 4,000 rpm for 5 minutes at 4oC.</LI> | ||
| + | <LI>Add 1 mL of chilled glycerol to each tube of culture.</LI> | ||
| + | <LI>Pipette up and down to mix it gently.</LI> | ||
| + | <LI>Add 100 ul culture in each eppendorf tube.</LI> | ||
| + | <LI>Store the culture at -80oC for approximately 1 hour.</LI> | ||
| + | </OL> | ||
| + | |||
| + | |- | ||
| + | |style="width:900px;font-size: 1.3em;"|Spread plate | ||
| + | |- | ||
| + | |style="width:900px;| | ||
| + | <OL> | ||
| + | <LI>Pipetting the liquid culture (about 200 ul) onto the surface of the LB agar plate.</LI> | ||
| + | <LI>Sterilize an L-shape glass rod.</LI> | ||
| + | <LI>Spread the cells evenly on the plate.</LI> | ||
| + | <LI>Incubate the plate at 37oC overnight with the bottom facing upward.</LI> | ||
| + | </OL> | ||
| + | |||
| + | |- | ||
| + | |style="width:900px;font-size: 1.3em;"|Streak plate | ||
| + | |- | ||
| + | |style="width:900px;| | ||
| + | <OL> | ||
| + | <LI>Sterilize the inoculating loop in flame.</LI> | ||
| + | <LI>Pick a portion of a single colony of the sample.</LI> | ||
| + | <LI>Make the first phase streak.</LI> | ||
| + | <LI>Flame sterilize the inoculation loop.</LI> | ||
| + | <LI>Cross the first phase of inoculum and make the second phase streak.</LI> | ||
| + | <LI>Repeat step d and e for making the third phase streak.</LI> | ||
| + | <LI>Flame sterilize the inoculating loop.</LI> | ||
| + | <LI>Incubate the plate at 37oC overnight with the bottom facing upward.</LI> | ||
| + | </OL> | ||
| + | |||
| + | |- | ||
| + | |style="width:900px;font-size: 1.3em;"|Transformation | ||
| + | |- | ||
| + | |style="width:900px;| | ||
| + | <OL> | ||
| + | <LI>Mix 1 ul of DNA in 100 ul of competent cells.</LI> | ||
| + | <LI>Place the mixture on ice for 40 minutes.</LI> | ||
| + | <LI>Heat shock the cells in 42oC water bath for 90 – 100 sec.</LI> | ||
| + | <LI>Incubate on ice for 3 minutes.</LI> | ||
| + | <LI>Recovery:</LI> | ||
| + | <OL> | ||
| + | <LI>Add 900 ul LB broth to the tube.</LI> | ||
| + | <LI>Incubate the mixture at 37oC for 1 hour in the rotary machine.</LI> | ||
| + | </OL> | ||
| + | <LI>Spread 100 ul of each culture on a LB agar plate.</LI> | ||
| + | <LI>Incubate at 37oC overnight.</LI> | ||
| + | </OL> | ||
| + | |||
| + | |- | ||
| + | |style="width:900px;font-size: 1.7em"|'''C. Preparation of materials''' | ||
| + | |- | ||
| + | |- | ||
| + | |style="width:900px;font-size: 1.3em;"|Preparation of ampicillin | ||
| + | |- | ||
| + | |style="width:900px;| | ||
| + | <OL> | ||
| + | <LI>Add 1 g of ampicillin powder to 10 mL of ddH2O. Mix well.</LI> | ||
| + | <LI>Filter the solution using 0.22 um filter.</LI> | ||
| + | <LI>Store the filtrate at -20 oC.</LI> | ||
| + | </OL> | ||
| + | |||
| + | |- | ||
| + | |style="width:900px;font-size: 1.3em;"|Preparation of LB agar plate | ||
| + | |- | ||
| + | |style="width:900px;| | ||
| + | <OL> | ||
| + | <LI>Add 7 g of LB powder to 200 mL of ddH2O. Mix well.</LI> | ||
| + | <LI>Autoclave the solution.</LI> | ||
| + | <LI>Add 180 ul of ampicillin to 180 mL of molten agar, in a ratio of 1:1000 (if necessary).</LI> | ||
| + | <LI>Pour 10 mL agar solution per plate.</LI> | ||
| + | <LI>Let the agar solidify.</LI> | ||
| + | </OL> | ||
| + | |||
| + | |- | ||
| + | |style="width:900px;font-size: 1.3em;"|Preparation of LB broth | ||
| + | |- | ||
| + | |style="width:900px;| | ||
| + | <OL> | ||
| + | <LI>Add 4 g of LB powder to 200 mL of ddH2O. Mix well.</LI> | ||
| + | <LI>Autoclave the solution.</LI> | ||
| + | <LI>Add 200 ul of ampicillin to 200 mL of LB broth (1:1000).</LI> | ||
| + | </OL> | ||
| + | |||
| + | |- | ||
| + | |style="width:900px;font-size: 1.3em;"|Preparation of 1X TAE buffer | ||
| + | |- | ||
| + | |style="width:900px;| | ||
| + | <OL> | ||
| + | <LI>Add 50 mL of 50X TAE buffer.</LI> | ||
| + | <LI>Add 2.5 L of ddH2O.</LI> | ||
| + | </OL> | ||
| + | |||
| + | |- | ||
| + | |style="width:900px;font-size: 1.7em"|'''D. Protein Work''' | ||
| + | |- | ||
| + | |style="width:900px;font-size: 1.3em;"|Gel Shift Assays (Adopt from Promega) | ||
| + | |- | ||
| + | |style="width:900px;| | ||
| + | <OL> | ||
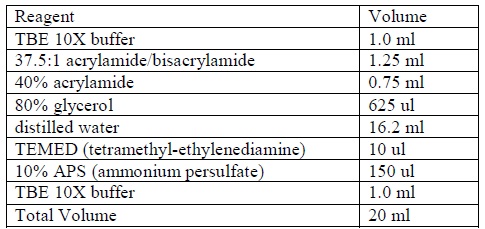
| + | <LI>Gel Preparation | ||
| + | <OL> | ||
| + | <LI>Clean all the glassware by distilled water (ions-free). | ||
| + | <LI>Prepare a non-denaturing 4% acrylamide gel according to the following formula: | ||
| + | <div ALIGN=CENTER> | ||
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Gel_shift_assay_gel_preparation.jpg|400px]] | ||
| + | |- | ||
| + | |Formula for preparing acrylamide gel. | ||
| + | |} | ||
| + | </div> | ||
| + | <LI>Allow the gel to stand until the gel is completely polymerized. | ||
| + | </OL> | ||
| + | |||
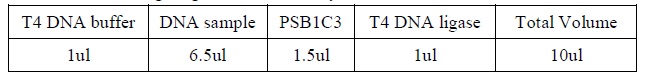
| + | <LI>DNA Binding Reactions | ||
| + | <OL> | ||
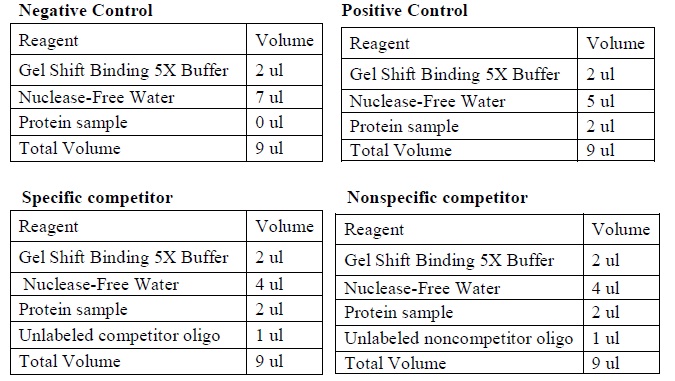
| + | <LI>Set up four binding reactions (if necessary) with the following composition. | ||
| + | <div ALIGN=CENTER> | ||
| + | {| style="width:254px;background:#99EE63;text-align:center;font-family: georgia, helvetica, arial, sans-serif;color:#000000;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:Gel_shoft_assay_DNA_binding_reactions.jpg|500px]] | ||
| + | |- | ||
| + | |The four binding reactions: Negative control, Positive control, Specific competitor and Non-specific competitor | ||
| + | |} | ||
| + | </div> | ||
| + | <LI>Incubate the reactions at room temperature for 10 minutes. | ||
| + | <LI>Add 1 ul of labeled DNA sample to each reaction. | ||
| + | <LI>Incubate the reactions at room temperature for 20 minutes. | ||
| + | <LI>Add 1 ul of 10X loading buffer for each reaction. | ||
| + | </OL> | ||
| + | |||
| + | <LI>Electrophoresis | ||
| + | <OL> | ||
| + | <LI>Pre-run the gel in 0.5X TBE buffer for 10 minutes at 350V. | ||
| + | <LI>Load the sample. | ||
| + | <LI>Run the gel at 350V until the loading dye reached three fourth of the gel. | ||
| + | <LI>Maintain the gel temperature under 30oC. | ||
| + | </OL> | ||
| + | </OL> | ||
| + | |||
| + | <LI>Protocol adopt from: | ||
| + | |||
| + | <LI>http://www.promega.com/~/media/Files/Resources/Protocols/Technical%20Bulletins/0/Gel%20Shift%20Assay%20Systems.ashx | ||
Latest revision as of 03:19, 5 October 2011
| Lab Protocol |
| A. DNA WORK |
| Agarose Gel Electrophoresis |
|
| DNA Extraction from Agarose Gel |
|
| DNA Digestion |
|
| Miniprep(Adopt from Qiagen) |
|
| Polymerase Chain Reaction |
|
| DNA ligation |
|
| Sequencing |
|
| B. BACTERIAL WORK |
| Overnight culture |
|
| Preparation of competent cell |
|
| Spread plate |
|
| Streak plate |
|
| Transformation |
|
| C. Preparation of materials |
| Preparation of ampicillin |
|
| Preparation of LB agar plate |
|
| Preparation of LB broth |
|
| Preparation of 1X TAE buffer |
|
| D. Protein Work |
| Gel Shift Assays (Adopt from Promega) |
|
 "
"