Team:UTP-Panama/Week 12
From 2011.igem.org
m (→GENERAL MEETING SESSION) |
(→WET LAB) |
||
| (23 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
<br> | <br> | ||
==Week 12: August 22 to 27== | ==Week 12: August 22 to 27== | ||
| - | |||
| - | |||
| - | |||
==August 22== | ==August 22== | ||
===WET LAB=== | ===WET LAB=== | ||
| - | ( | + | '''Preparation of LB''' |
| - | + | ||
| + | We prepared additional 15 mL LB to continue activities from Aug 19, 2011 | ||
| + | calculation: <br> | ||
| + | 15 mL de LB × (33μg Chloramphenicol)/(mL de LB) × mL/(34mg Chloramphenicol)= 14μL <br> | ||
| + | We inoculate and incubate the petri dishes | ||
==August 23== | ==August 23== | ||
===WET LAB=== | ===WET LAB=== | ||
| - | + | We did a dirty miniprep to extract plasmidic DNA of the Biobricks <br> | |
| - | + | • BBa_K410000, pSB1C3 <br> | |
| + | • BBa_K328003, pSB1C3 <br> | ||
| + | • BBa_K328001, pSB1C3 <br> | ||
| + | And stored until next day <br> | ||
==August 24== | ==August 24== | ||
===WET LAB=== | ===WET LAB=== | ||
| - | + | Electrophoresis was applied to the following BioBricks: <br> | |
| - | + | ||
| - | + | • BBa_K410000, pSB1C3 <br> | |
| - | + | • BBa_K328003, pSB1C3 <br> | |
| - | + | • BBa_K328001, pSB1C3 <br> | |
| - | + | ||
| - | + | 1. Preparation of agarose 1%: <br> | |
| - | + | In a 250mL Erlenmeyer we mixed 0.5 g agarose with 50 mL of 1X TAE (for electrophoresis gel). <br> | |
| - | ( | + | 2. We heated in a microwave to dissolve (5 times of 20s each time stirring gently). <br> |
| - | + | 3. We cooled in a stream of gently flowing water. <br> | |
| + | 4. We added 1,5 µL of ethidium bromide by touching the liquid. Stir until dissolved. <br> | ||
| + | 5. We added the blend to the electrophoresis cell with the combo on it. When the gel solidified we removed the comb and the wells were marked in the gel. <br> | ||
| + | 6. We placed part of the cell where the gel was solidified in the cell and filled it with TAE 1X. <br> | ||
| + | 7. We put on a piece of parafilm 10 drops of 2 μL c / u indicator LB 6X. <br> | ||
| + | 8. We placed in parafilm 2 µL of Supercoiled DNA Ladder reference. <br> | ||
| + | 9. We extracted 5 µL of an eppendorf and resuspended in LB 6X on parafilm. <br> | ||
| + | 10. Placed samples in the wells gell. <br> | ||
| + | 11. We repeated steps 9 and 10 until the contents of each eppendorf was placed in each well. <br> | ||
| + | 12. We resuspended 2 µL of supercoiled DNA ladder 2μL with a drop (2μL) LB 6X and we placed in the correspondant well. <br> | ||
| + | 13. We put the electrodes so that the samples ran from the negative to the positive terminal of the cell. | ||
| + | 14. We ran the electrophoresis for about two hours. | ||
| + | |||
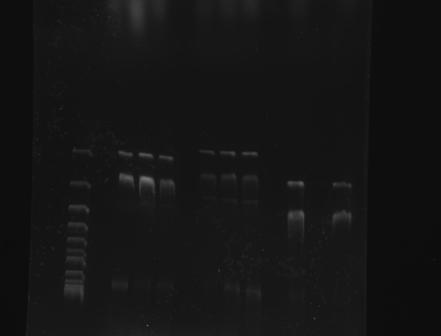
| + | Gel Electrophoresis of Part:BBa_K410000 and the CspA Promoter <br> | ||
| + | 1 2 3 4 5 6 7 8 9 10 | ||
| + | [[File:GATECH, UNAM 003 Y 001 TOMA2 24AGO11.jpg]] | ||
| + | |||
| + | 1. Reference (DNA Supercoiled Ladder) | ||
| + | |||
| + | 2, 3, 4 : BBa_K328001 | ||
| + | |||
| + | 5, 6, 7 : BBa_K328003 | ||
| + | |||
| + | 8, 9, 10: BBa_K410000 | ||
==August 27== | ==August 27== | ||
Latest revision as of 04:57, 29 September 2011
|
Home |
Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 | Week 10 | Week 11 | Week 12 | Week 13 | Week 14 | Week 15 | Week 16 | Week 17 | After Regional Week 1 | After Regional Week 2 |
Week 12: August 22 to 27August 22WET LABPreparation of LB We prepared additional 15 mL LB to continue activities from Aug 19, 2011
calculation: August 23WET LABWe did a dirty miniprep to extract plasmidic DNA of the Biobricks August 24WET LABElectrophoresis was applied to the following BioBricks: • BBa_K410000, pSB1C3 1. Preparation of agarose 1%: Gel Electrophoresis of Part:BBa_K410000 and the CspA Promoter 1 2 3 4 5 6 7 8 9 10 1. Reference (DNA Supercoiled Ladder) 2, 3, 4 : BBa_K328001 5, 6, 7 : BBa_K328003 8, 9, 10: BBa_K410000 August 27GENERAL MEETING SESSIONSAFETY DUE HUMAN PRACTICE We also prepared details of our project and ongoing activies. Director of Session: Grimaldo E. Ureña & Lucia Palma. |
 "
"