Team:Arizona State/Notebook/August
From 2011.igem.org
(Difference between revisions)
| Line 14: | Line 14: | ||
* Submitted BMES abstract | * Submitted BMES abstract | ||
== Tuesday, August 2 == | == Tuesday, August 2 == | ||
| - | * | + | * Transformation results from yesterday: |
:* 4 colonies on seq1 | :* 4 colonies on seq1 | ||
:* DA colonies all clumped together | :* DA colonies all clumped together | ||
:* RA fail, among stacks of failed transformations 3: | :* RA fail, among stacks of failed transformations 3: | ||
| - | * | + | * New RLT for 2x |
:* Varying DNA volumes added (1, 2.5, and 5 uL RLTs) | :* Varying DNA volumes added (1, 2.5, and 5 uL RLTs) | ||
* PCR: | * PCR: | ||
| - | :* | + | :* Seq1, RA, DA in puc57 using puc57 primers |
| - | ::* | + | ::* PCR settings: from previous |
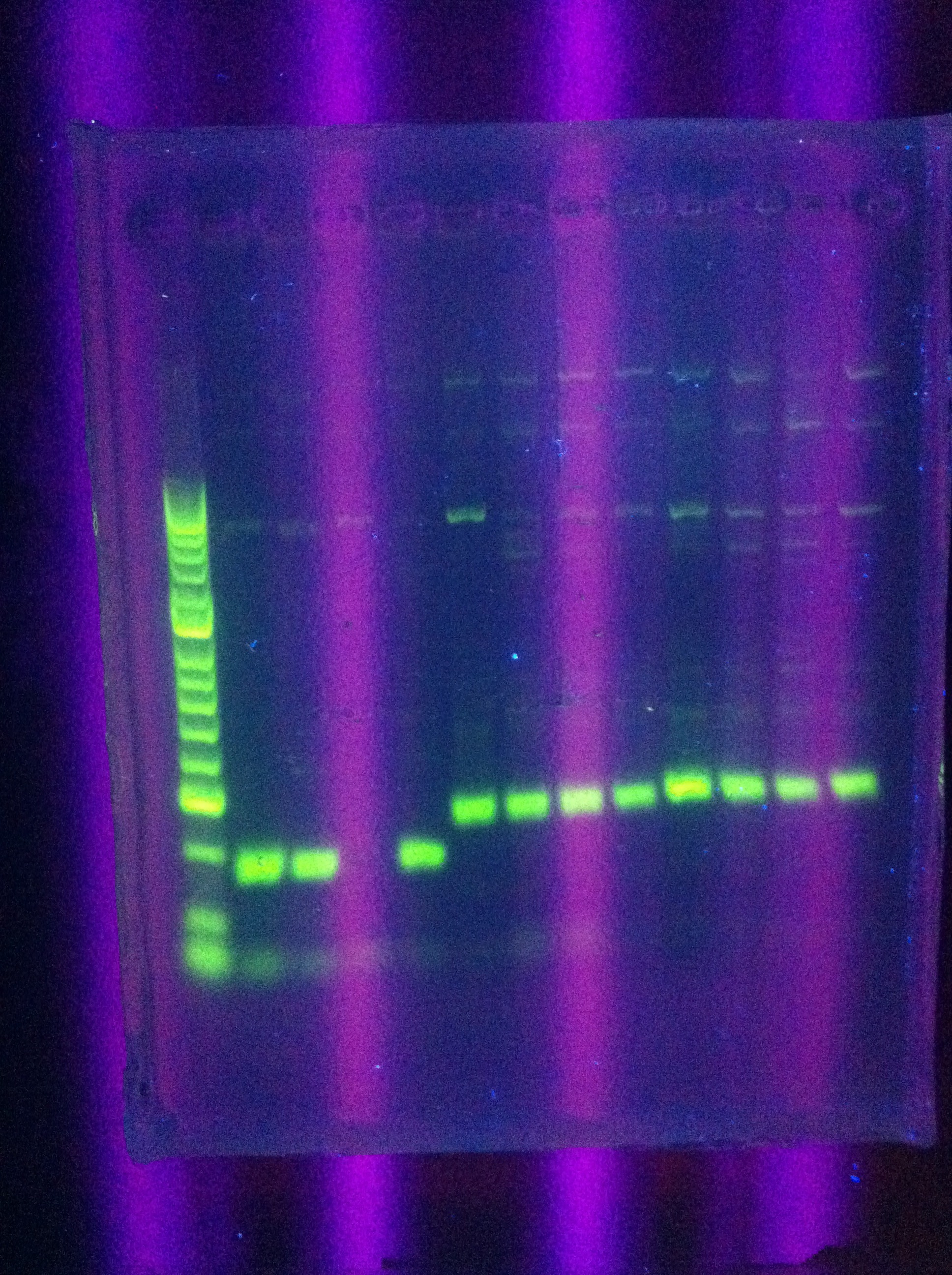
* Gel results: look the same as before | * Gel results: look the same as before | ||
<br> [[Image:ASU_82_IMG_3989.jpg|400px]] | <br> [[Image:ASU_82_IMG_3989.jpg|400px]] | ||
| - | :* | + | :* Clear bands ~100 for Seq1 |
:* ~200 for DA, RA | :* ~200 for DA, RA | ||
| - | * | + | * Submitted for sequencing: |
| - | :* BAHHH jason | + | :* BAHHH jason (sequencing delays) |
:* Resubmitted 1x Seq1, RA, DA(pcr and prep), GFP, RFP, Cas3 | :* Resubmitted 1x Seq1, RA, DA(pcr and prep), GFP, RFP, Cas3 | ||
* Streak plates | * Streak plates | ||
| Line 36: | Line 36: | ||
:* 2xSeq1 in psb1k3 | :* 2xSeq1 in psb1k3 | ||
:* BL21DE3 from glycerol stock | :* BL21DE3 from glycerol stock | ||
| - | ::* | + | ::* Test tube with larger stopper may be contaminated |
== Wednesday, August 3 == | == Wednesday, August 3 == | ||
* Liquid cultures | * Liquid cultures | ||
| Line 42: | Line 42: | ||
:* 1xDA | :* 1xDA | ||
* PCR | * PCR | ||
| - | :* | + | :* Cleanup of PCR amplified Seq1, RA, DA from puc57 |
| - | :* ( | + | :* (We gelled this last night, looked good) |
:* Used RA from this for RLT --> | :* Used RA from this for RLT --> | ||
* RLT | * RLT | ||
| Line 54: | Line 54: | ||
* ABCDE PCR with the newest primers (v3) | * ABCDE PCR with the newest primers (v3) | ||
:* 98deg initial denaturation for 30 seconds | :* 98deg initial denaturation for 30 seconds | ||
| - | :* | + | :* Cycle {10 sec at 98 degrees |
| - | ::* | + | ::* Anneal at 63 degrees for 30 sec |
| - | ::* | + | ::* Elongate at 72 degrees for 130 sec} |
:* 35 cycles | :* 35 cycles | ||
| - | :* | + | :* Then extension for 450 seconds |
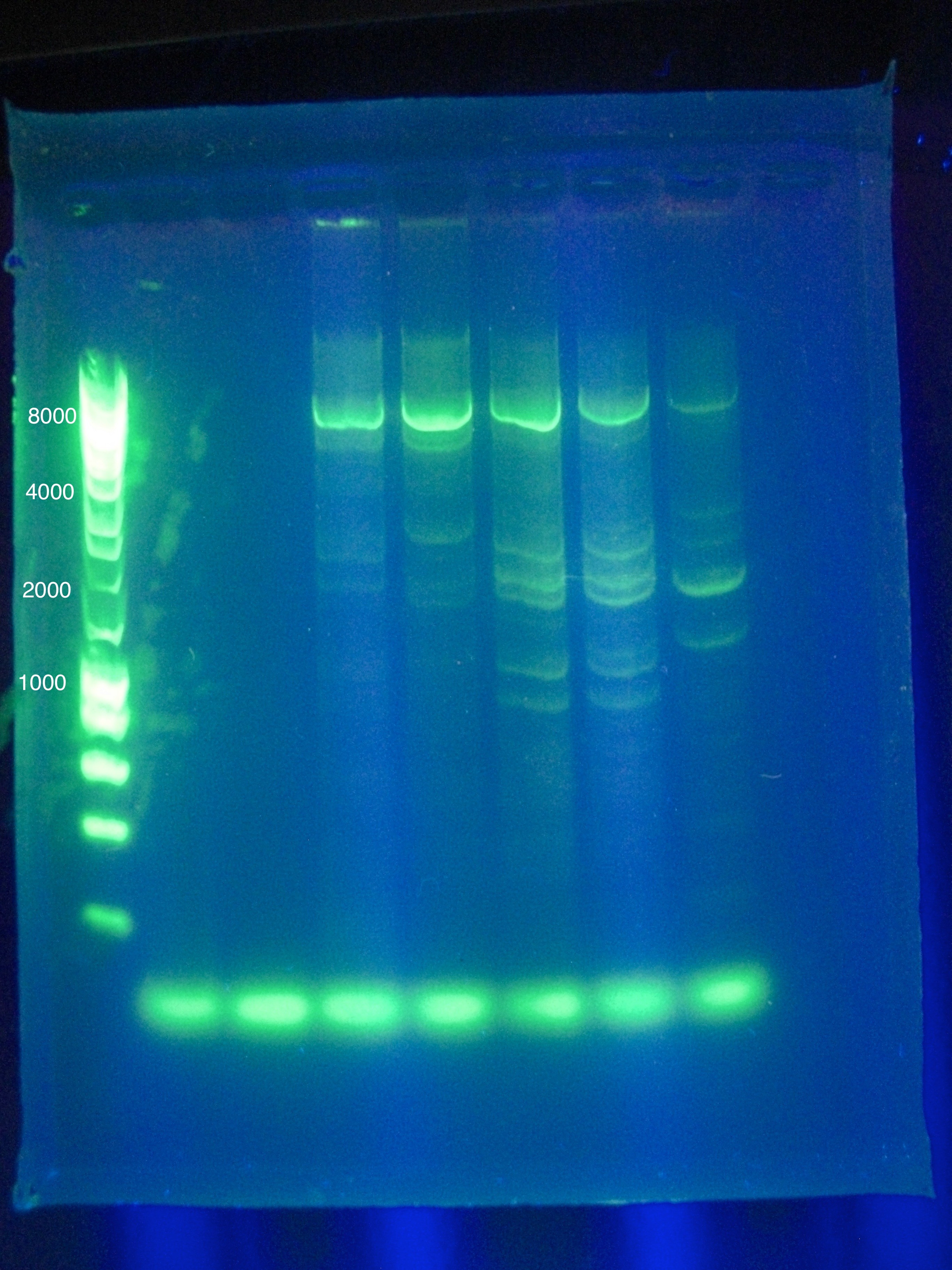
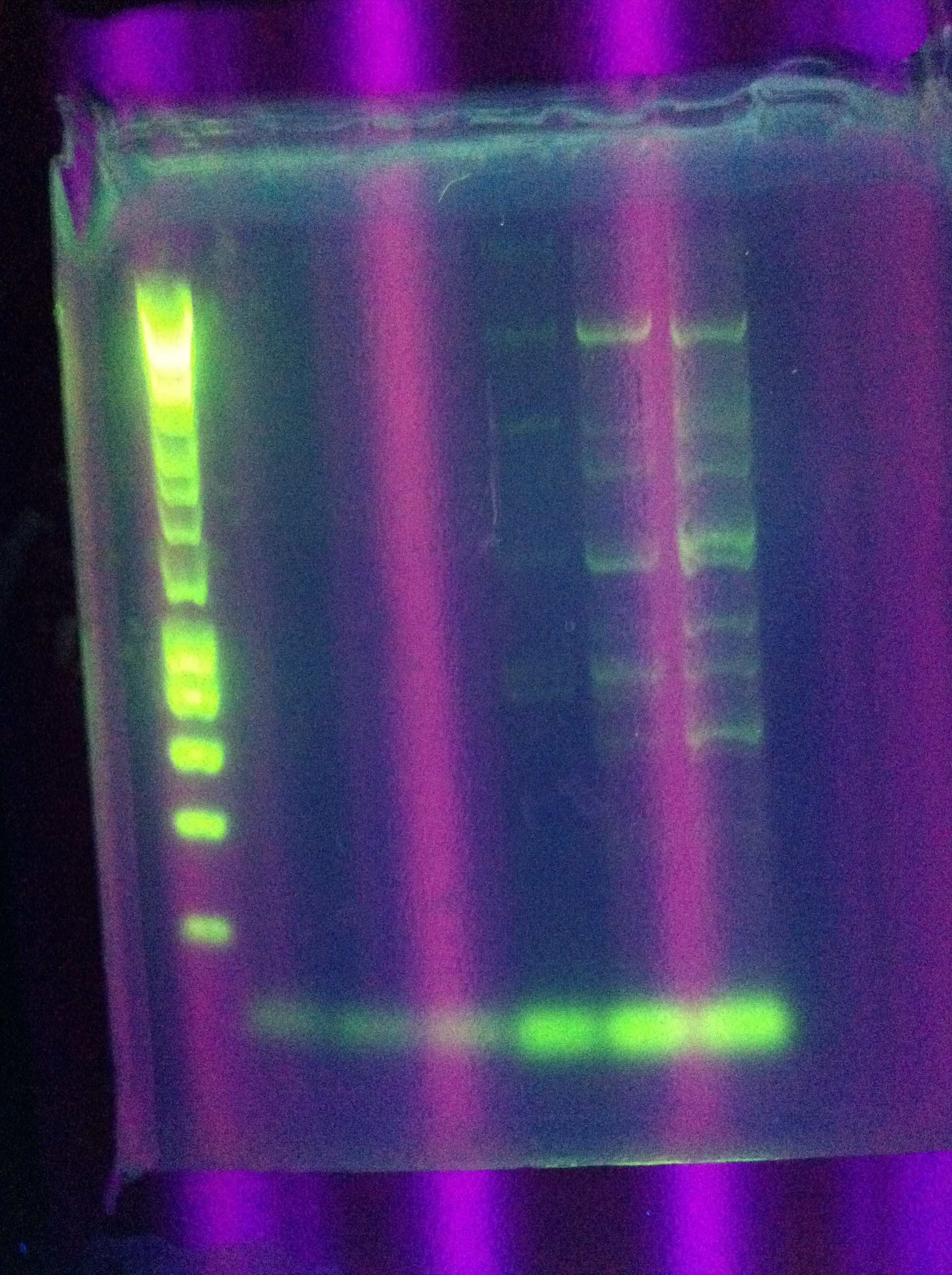
* Results show clearly a band above where we would like it, at around 8kbp, and also some bands below the desired length at around 2kbp | * Results show clearly a band above where we would like it, at around 8kbp, and also some bands below the desired length at around 2kbp | ||
<br> [[Image:ASU_84_Gel_for_PCR.jpg|400px]] | <br> [[Image:ASU_84_Gel_for_PCR.jpg|400px]] | ||
<br> [[Image:ASU_84_IMG_3998.jpg|400px]] | <br> [[Image:ASU_84_IMG_3998.jpg|400px]] | ||
== Friday, August 5 == | == Friday, August 5 == | ||
| - | * | + | * Miniprep: |
| - | :* | + | :* Using old protocol |
:* RAGE, SEGE, RFP overnight cultures | :* RAGE, SEGE, RFP overnight cultures | ||
| - | * | + | * Gel: |
:* PCR from last night | :* PCR from last night | ||
| - | ::* | + | ::* CasABCDE (upper): |
| - | :::* | + | :::* See photo |
| - | :::* | + | :::* Band > 10k, genomic? |
| - | ::* | + | ::* CasABCDE (lower): |
| - | :::* | + | :::* Same |
| - | * | + | * Gel extraction of PCR 8-4 (see photo) |
* PCR of gel extraction CasABCDE: | * PCR of gel extraction CasABCDE: | ||
| - | :* | + | :* Sample 1,2,3 |
* Primers: CasABcDE_V3 F/R | * Primers: CasABcDE_V3 F/R | ||
:* Thermocycle: ABCDE802 | :* Thermocycle: ABCDE802 | ||
:* Template: gel extraction sample 1 of PCR 8-4 (above) | :* Template: gel extraction sample 1 of PCR 8-4 (above) | ||
* Mg2+ gradient | * Mg2+ gradient | ||
| - | :* | + | :* Sample1: 0 mM |
| - | :* | + | :* Sample2: 1mM |
| - | :* | + | :* Sample3: 2mM |
:* Cycle strarted 5:30pm | :* Cycle strarted 5:30pm | ||
* Colony PCR: | * Colony PCR: | ||
| - | :* | + | :* Sample 1c, 2c,3c |
:* Primers: CasABcDE_V3 F/R | :* Primers: CasABcDE_V3 F/R | ||
* Thermocycle: ABCDE802 | * Thermocycle: ABCDE802 | ||
| Line 95: | Line 95: | ||
* New 10mM dNTP solution made from dNTP stock. | * New 10mM dNTP solution made from dNTP stock. | ||
* RLT: | * RLT: | ||
| - | :* | + | :* Todo |
== Monday, August 8 == | == Monday, August 8 == | ||
GROUP MEETING | GROUP MEETING | ||
| Line 135: | Line 135: | ||
:* Gloves | :* Gloves | ||
:* Plates | :* Plates | ||
| - | :* | + | :* T4 polymerase |
| - | :* | + | :* Miniprep |
| - | :* | + | :* Listeria innocua items |
== Tuesday, August 9 == | == Tuesday, August 9 == | ||
| - | * | + | * Compiled cas gene information to table on wiki |
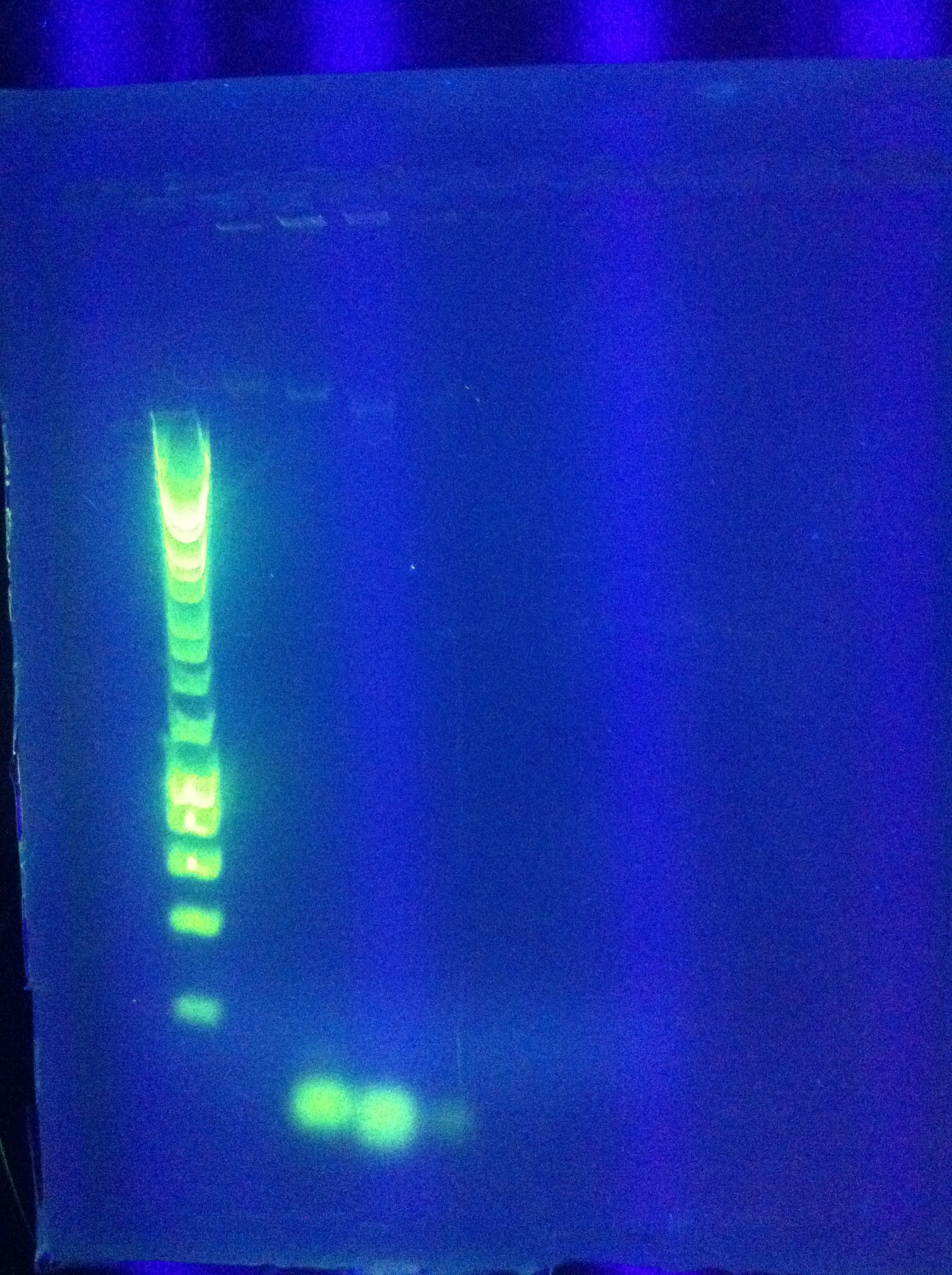
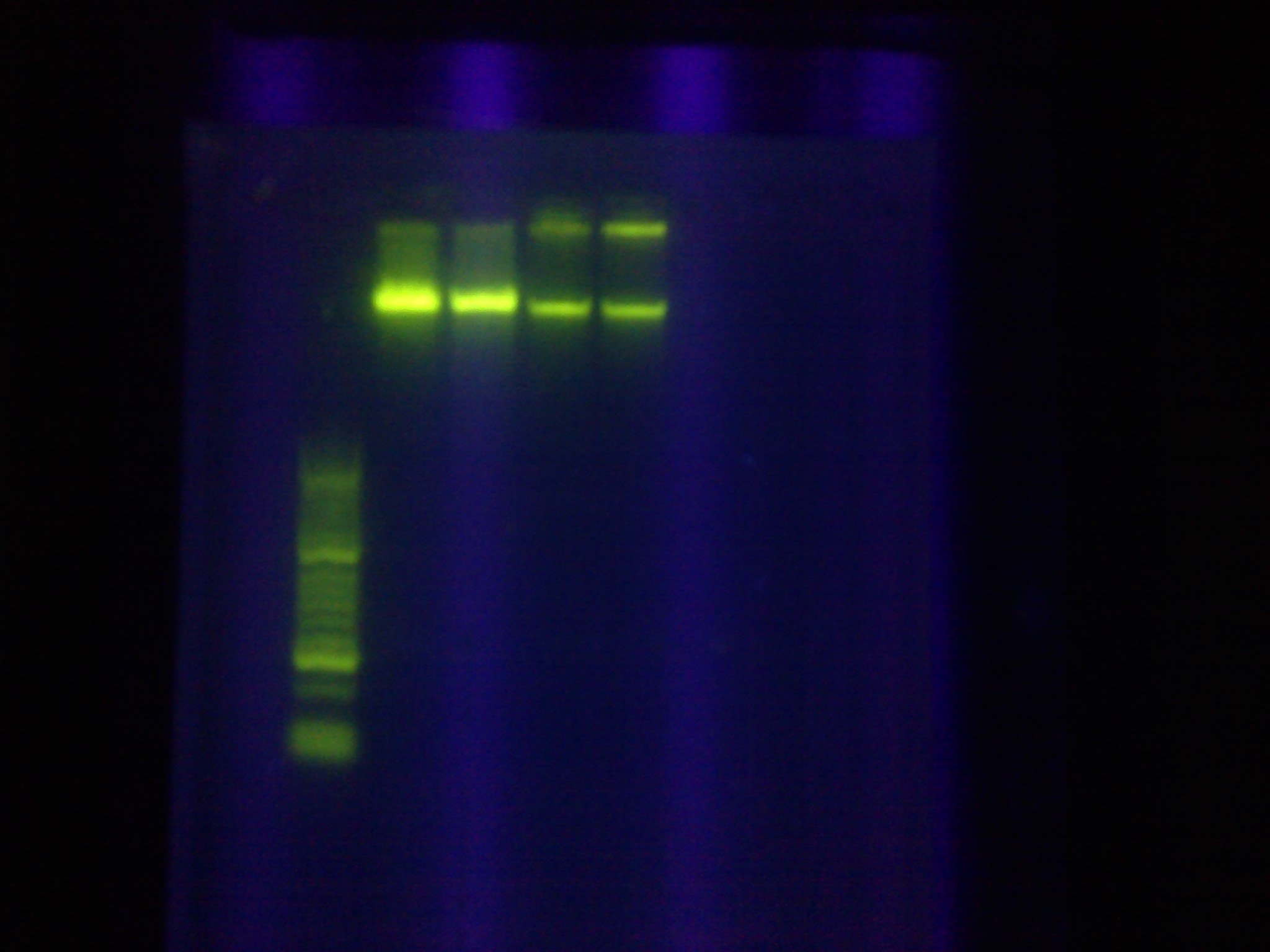
* Confirmed that RA was successfully amplified with a gel | * Confirmed that RA was successfully amplified with a gel | ||
<br> [[Image:ASU_89_IMG_4047.jpg|400px]] | <br> [[Image:ASU_89_IMG_4047.jpg|400px]] | ||
| Line 146: | Line 146: | ||
* Incubated restriction/ligation at 37 degrees overnight | * Incubated restriction/ligation at 37 degrees overnight | ||
* Liquid cultures from 8/8 failed to grow | * Liquid cultures from 8/8 failed to grow | ||
| - | :* | + | :* Potentially bad LB-Kan broth |
* Made new LB-Kan broth | * Made new LB-Kan broth | ||
* New liquid cultures of Seq1 and RA (2x, each) | * New liquid cultures of Seq1 and RA (2x, each) | ||
| - | :* | + | :* Old LB-Kan broth |
| - | :* | + | :* New LB-Kan broth |
TO DO for 8-10-11: | TO DO for 8-10-11: | ||
| - | * | + | * Gel RL results |
* PCR cleanup restriction/ligation results | * PCR cleanup restriction/ligation results | ||
| - | * | + | * Possibly PCR cleanup RA inserts from PCR amplification for re-restriction/re-ligation |
== Wednesday, August 10 == | == Wednesday, August 10 == | ||
* Liquid cultures from 8/9 all failed. | * Liquid cultures from 8/9 all failed. | ||
| - | :* | + | :* Both old and new LB+Kan broth |
* Positive and negative controls started | * Positive and negative controls started | ||
| - | :* | + | :* Old and new LB-Kan broth |
* RA and Seq1 from 8/8 streak plated | * RA and Seq1 from 8/8 streak plated | ||
* Liquid cultures in LB-Amp broth started | * Liquid cultures in LB-Amp broth started | ||
| - | :* | + | :* From pUC57, RA and Seq1 |
:* 2 liquid cultures of each | :* 2 liquid cultures of each | ||
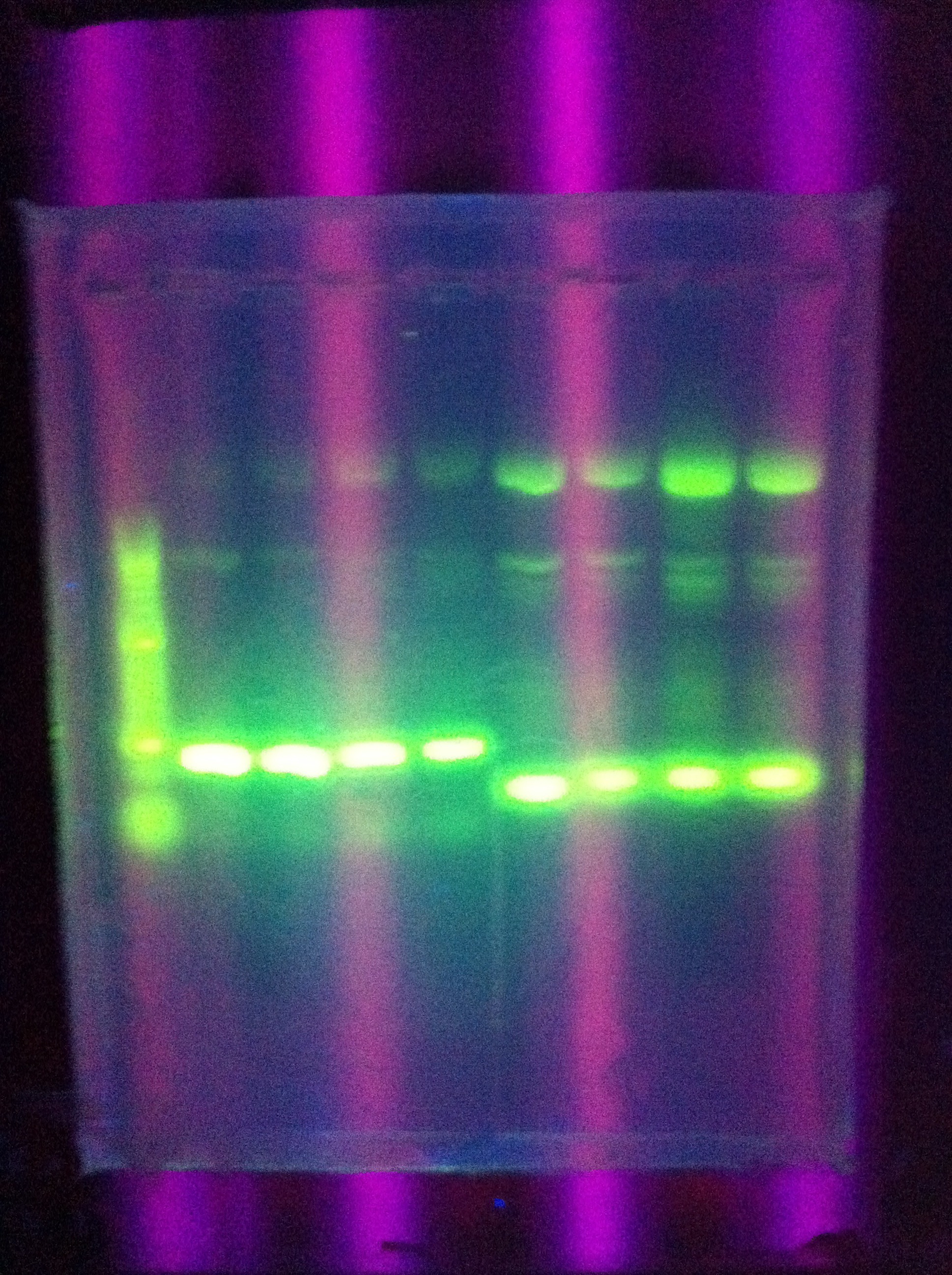
* Gel results for last night's PCR of Cas3 (see pic) | * Gel results for last night's PCR of Cas3 (see pic) | ||
| Line 188: | Line 188: | ||
* Ran a gel of last night's PCR of ABCDE (see pic) | * Ran a gel of last night's PCR of ABCDE (see pic) | ||
:* DMSO worked -- same results as previously but less overall bands | :* DMSO worked -- same results as previously but less overall bands | ||
| - | :* | + | :* Seemed to hone in on the band that is above our desired length (~8k or so) |
* (For reference to the picture, we ran some kind of restriction product that I can't recall on the right side w/ Hyperladder II) | * (For reference to the picture, we ran some kind of restriction product that I can't recall on the right side w/ Hyperladder II) | ||
:* Does anyone remember this? Might be Restricted RB | :* Does anyone remember this? Might be Restricted RB | ||
| Line 238: | Line 238: | ||
:* PCR amplification of Seq1, RA | :* PCR amplification of Seq1, RA | ||
::* 14 tubes total (7 each at null, 0, 1, 2, 4, 8, 10) | ::* 14 tubes total (7 each at null, 0, 1, 2, 4, 8, 10) | ||
| - | :* | + | :* Note we are running out of Phusion already! |
* Another PCR (Madeline and Kylie) | * Another PCR (Madeline and Kylie) | ||
:* Using template from earlier PCR | :* Using template from earlier PCR | ||
| Line 247: | Line 247: | ||
::* Same as ABCDE808 but 35 cycles total (2:10 elongation time, 63deg annealing temp) | ::* Same as ABCDE808 but 35 cycles total (2:10 elongation time, 63deg annealing temp) | ||
== Saturday, August 13 == | == Saturday, August 13 == | ||
| - | * | + | * Restriction on poly x to remove incorrectly oriented pieces |
* Gel results for Taq Polymerase try at Cas3 (see picture) | * Gel results for Taq Polymerase try at Cas3 (see picture) | ||
:* Old primers: nonspecific all the way down | :* Old primers: nonspecific all the way down | ||
| Line 257: | Line 257: | ||
:* Miniprep of leader sequences & nanodrops (maybe this was Friday) | :* Miniprep of leader sequences & nanodrops (maybe this was Friday) | ||
== Sunday, August 14 == | == Sunday, August 14 == | ||
| - | * | + | * Genome prep MG1655 |
== Monday, August 15 == | == Monday, August 15 == | ||
Team meeting | Team meeting | ||
| Line 283: | Line 283: | ||
:* BL21 DE3 for more TSS cells | :* BL21 DE3 for more TSS cells | ||
:* Keith's 2x RA and Seq1 streak plates from 8/15 (grew this time!) | :* Keith's 2x RA and Seq1 streak plates from 8/15 (grew this time!) | ||
| - | ::* | + | ::* Used LB-Kan broth from 8/11 (made by Abhi) |
* PCR amplification of Seq1 in pUC57 | * PCR amplification of Seq1 in pUC57 | ||
:* Gel visualized, ~150bp bands in samples 4,8,10 | :* Gel visualized, ~150bp bands in samples 4,8,10 | ||
| - | ::* | + | ::* Still on transilluminator |
| - | :* | + | :* Samples labeled and in -20 |
* PCR log updated | * PCR log updated | ||
* New PCR of Cas genes | * New PCR of Cas genes | ||
| Line 304: | Line 304: | ||
::* Might just use 8/13 tube as template, try again | ::* Might just use 8/13 tube as template, try again | ||
== Wednesday, August 17 == | == Wednesday, August 17 == | ||
| - | * | + | * Made tss cells (bl21, mg1655) |
| - | * | + | * Genome prep: mg1655 |
* 2 Minipreps of the K12 Leader and the B. Halo Leader. | * 2 Minipreps of the K12 Leader and the B. Halo Leader. | ||
== Thursday, August 18 == | == Thursday, August 18 == | ||
| Line 335: | Line 335: | ||
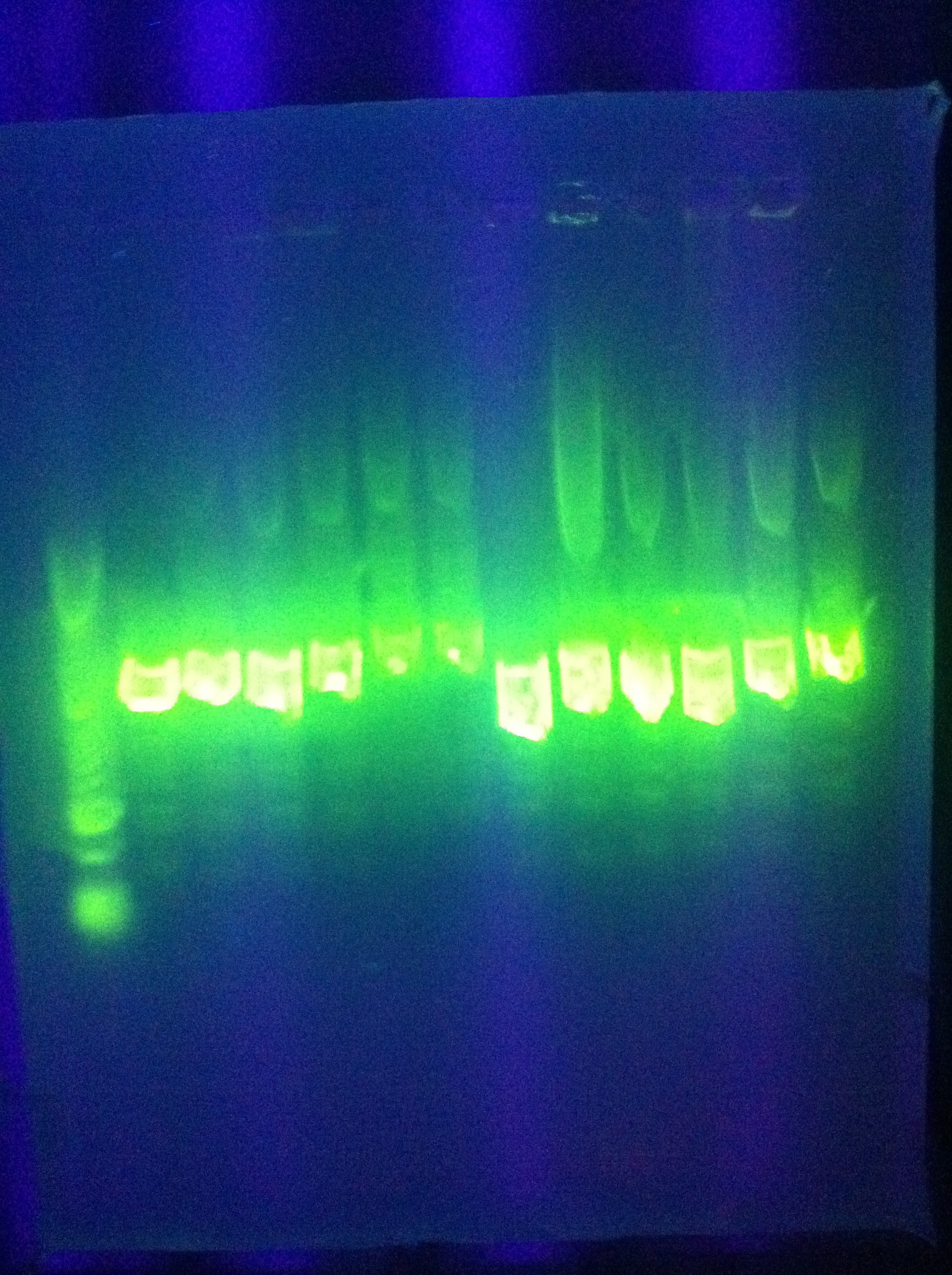
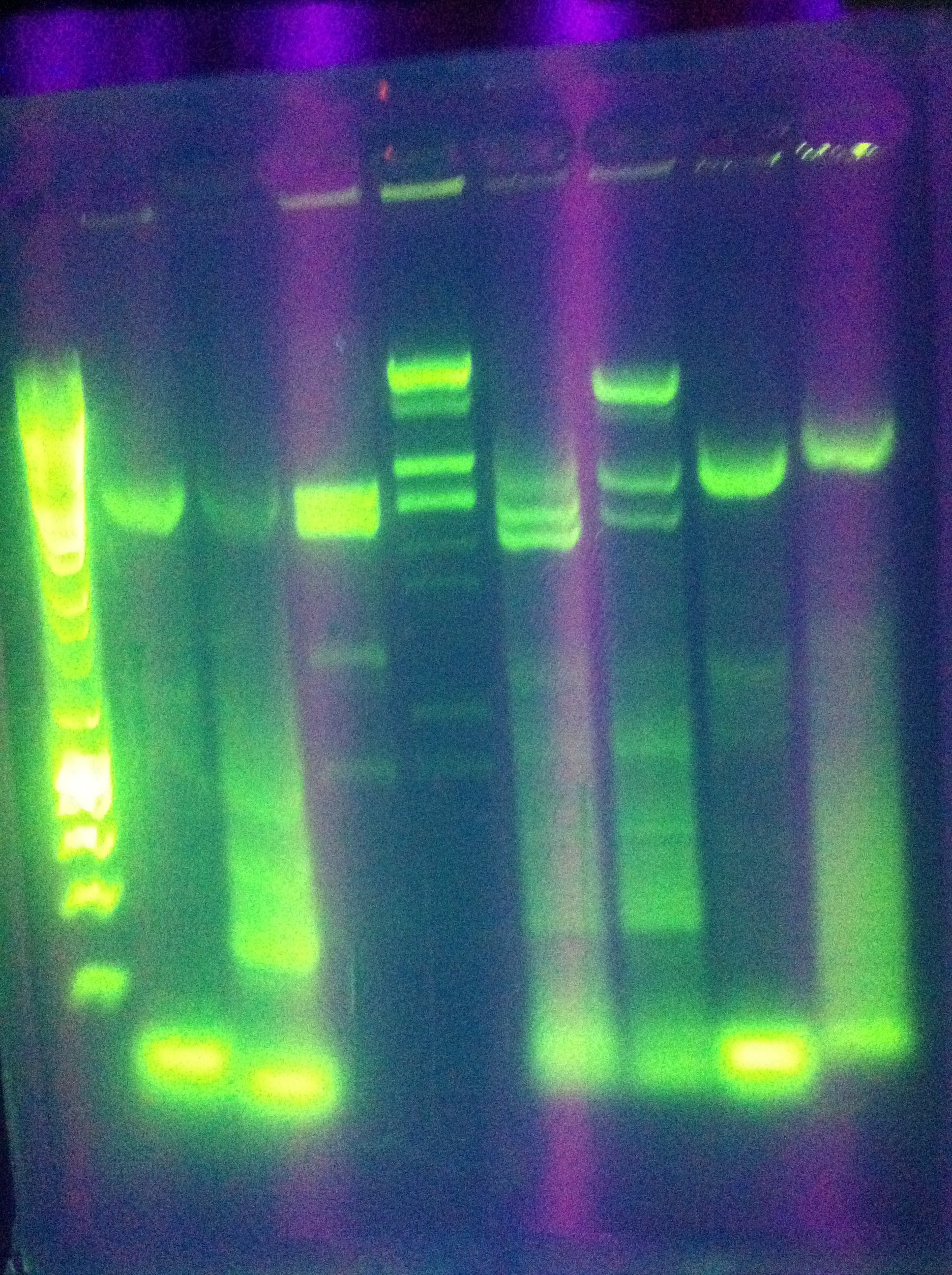
* Diagnostic gel ran of GFP and RFP cut. GFP shows slight degradation, with less linearized plasmid than expected. RFP backbone not visible. Seq1-1 PCR product from 8-19 is present at ~200bp. GFP in 1A3 and RFP in 1K3 on the DAN/Abhi tray do not pass. | * Diagnostic gel ran of GFP and RFP cut. GFP shows slight degradation, with less linearized plasmid than expected. RFP backbone not visible. Seq1-1 PCR product from 8-19 is present at ~200bp. GFP in 1A3 and RFP in 1K3 on the DAN/Abhi tray do not pass. | ||
:* Lane 1: Hyperladder 1 | :* Lane 1: Hyperladder 1 | ||
| - | :* | + | :* Lane 2: GFP in pSB1A3 |
| - | :* | + | :* Lane 3: GFP cut with EcorI and PstI |
| - | :* | + | :* Lane 4: RFP in pSB1K3 |
| - | :* | + | :* Lane 5: RFP cut with EcorI and PstI |
| - | :* | + | :* Lane 6: K12 Leader 2 (from 8-18) |
| - | :* | + | :* Lane 7: K12 Leader 4 (From 8-18) |
| - | :* | + | :* Lane 8: Seq1 PCR 1(From 8-19) |
* Restrictions of RA and Seq1 | * Restrictions of RA and Seq1 | ||
:* Samples: (Stored at -20 on top shelf "1-5 KSB") | :* Samples: (Stored at -20 on top shelf "1-5 KSB") | ||
| Line 353: | Line 353: | ||
* Coupled Restriction Ligation Attempt | * Coupled Restriction Ligation Attempt | ||
:* Sample incubated for 1 hour at 25 degrees then 8uL was ran on diagnostic gel. Sample shows nebulization at ~400 bp, this may be nuclease contamination but may not be. It may be a mixture of products forming from multiple ligations. Or it may be an unknown nuance inhibiting mutual restriction and ligation (See image: 8-20 1_2_3_4_5_RE+Lig). Note: the sample may need to be restricted, then column prepared using the Sigma-Aldrich PCR Cleanup Kit (Which appears to more effectively remove small <100nt fragments than Quiagen kit), then attempt coupled restriction ligation. If nebulazation still appears the reaction concept may be flawed. | :* Sample incubated for 1 hour at 25 degrees then 8uL was ran on diagnostic gel. Sample shows nebulization at ~400 bp, this may be nuclease contamination but may not be. It may be a mixture of products forming from multiple ligations. Or it may be an unknown nuance inhibiting mutual restriction and ligation (See image: 8-20 1_2_3_4_5_RE+Lig). Note: the sample may need to be restricted, then column prepared using the Sigma-Aldrich PCR Cleanup Kit (Which appears to more effectively remove small <100nt fragments than Quiagen kit), then attempt coupled restriction ligation. If nebulazation still appears the reaction concept may be flawed. | ||
| - | + | ||
| - | + | ||
| - | + | ||
== Wednesday, August 24 == | == Wednesday, August 24 == | ||
* Minipreps: | * Minipreps: | ||
| Line 414: | Line 412: | ||
:* Samples 1-6, 2-28 | :* Samples 1-6, 2-28 | ||
* Note: All eluted in kit Elution Buffer. | * Note: All eluted in kit Elution Buffer. | ||
| - | + | ||
| - | + | ||
== Wednesday, August 31 == | == Wednesday, August 31 == | ||
* Samples (Primer): Result Summary | * Samples (Primer): Result Summary | ||
Latest revision as of 03:06, 29 September 2011
|
|
Monday, August 1
Tuesday, August 2
Wednesday, August 3
Thursday, August 4
Friday, August 5
Monday, August 8GROUP MEETING
Tuesday, August 9
TO DO for 8-10-11:
Wednesday, August 10
Thursday, August 11
Friday, August 12
Saturday, August 13
Sunday, August 14
Monday, August 15Team meeting
Tuesday, August 16
Wednesday, August 17
Thursday, August 18
Friday, August 19
Saturday, August 20
Incubated for 2 hours at 37 degrees.
Wednesday, August 24
Thursday, August 25
Friday, August 26
Saturday, August 27
Sunday, August 28
Wednesday, August 31
|
 "
"