Team:Arizona State/Notebook/July
From 2011.igem.org
(Difference between revisions)
| (4 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
* Conducted miniprep | * Conducted miniprep | ||
* Conducted restriction: | * Conducted restriction: | ||
| - | :* Seq1, DA, RA (ES, EX) in | + | :* Seq1, DA, RA (ES, EX) in PSB1A3 |
* Conducted ligation attempt to make tandem RSR, RS, and SR constructs: | * Conducted ligation attempt to make tandem RSR, RS, and SR constructs: | ||
| - | :* Seq1 + Seq1, DA + DA, RA + RA | + | :* Seq1 + Seq1, DA + DA, RA + RA; all into pSB1K3 |
| - | :* Transformation of ligation products, plated on Kan (NEB cells) | + | :* Transformation of ligation products, plated on LB+Kan (NEB cells) |
| - | * ligation: | + | * Also, another conducted ligation: |
| - | :* | + | :* Seq1 (ES, XP) + PSB1A3 (EP) |
| - | :* | + | :* Transformation, plated on LB+Amp (NEB cells) |
| - | * glycerol stocks of | + | * Made glycerol stocks of Seq1, DA, DB, RA, RB in PSB1K3 |
== Wednesday, July 6 == | == Wednesday, July 6 == | ||
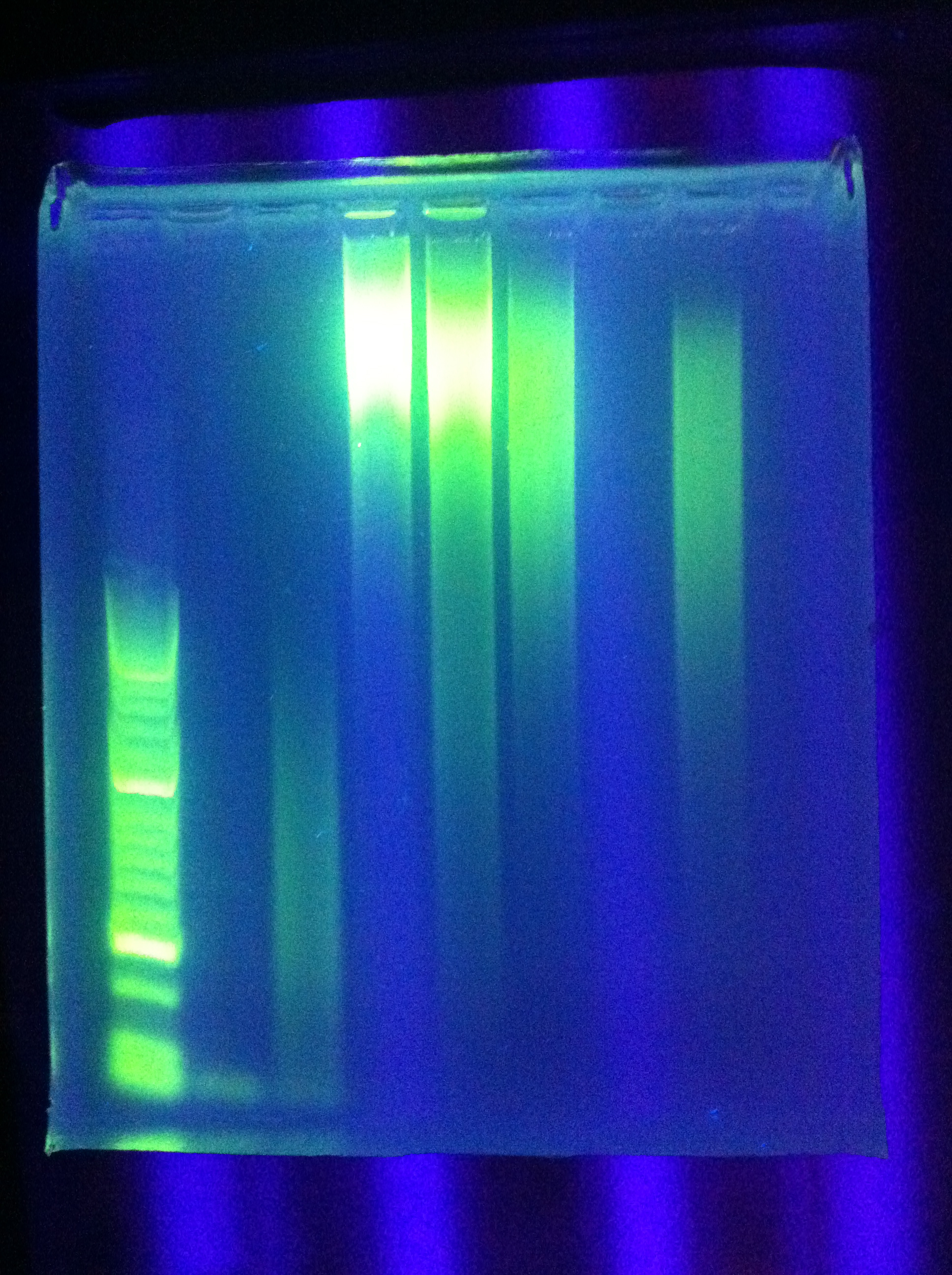
| - | * transformation results: | + | * Observed transformation results: |
| - | :* | + | :* Kan plates: |
| - | ::* | + | ::* All successful (?) (Seq1 + Seq1, DA + DA, RA + RA), will be sequenced (ordered biobrick forward / reverse primers) |
| - | :::* | + | :::* Made liquid culture for miniprep tomorrow |
| - | :* | + | :* Amp plates: |
| - | ::* | + | ::* No successes |
* PCR: | * PCR: | ||
| - | :* | + | :* Cas_b, Cas_c both unsuccessful |
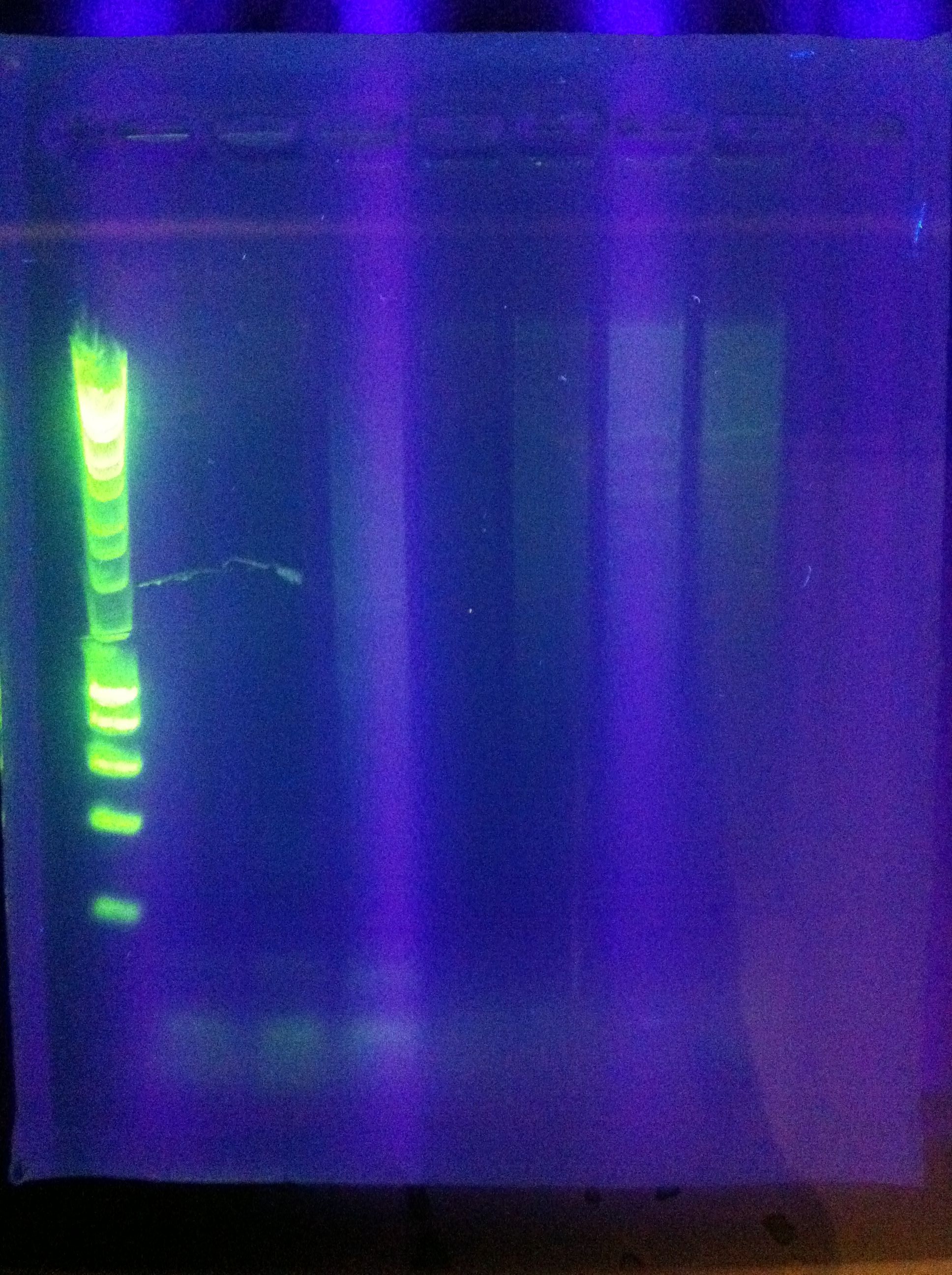
<br> [[Image:ASU_76_gel1.jpg|400px]] | <br> [[Image:ASU_76_gel1.jpg|400px]] | ||
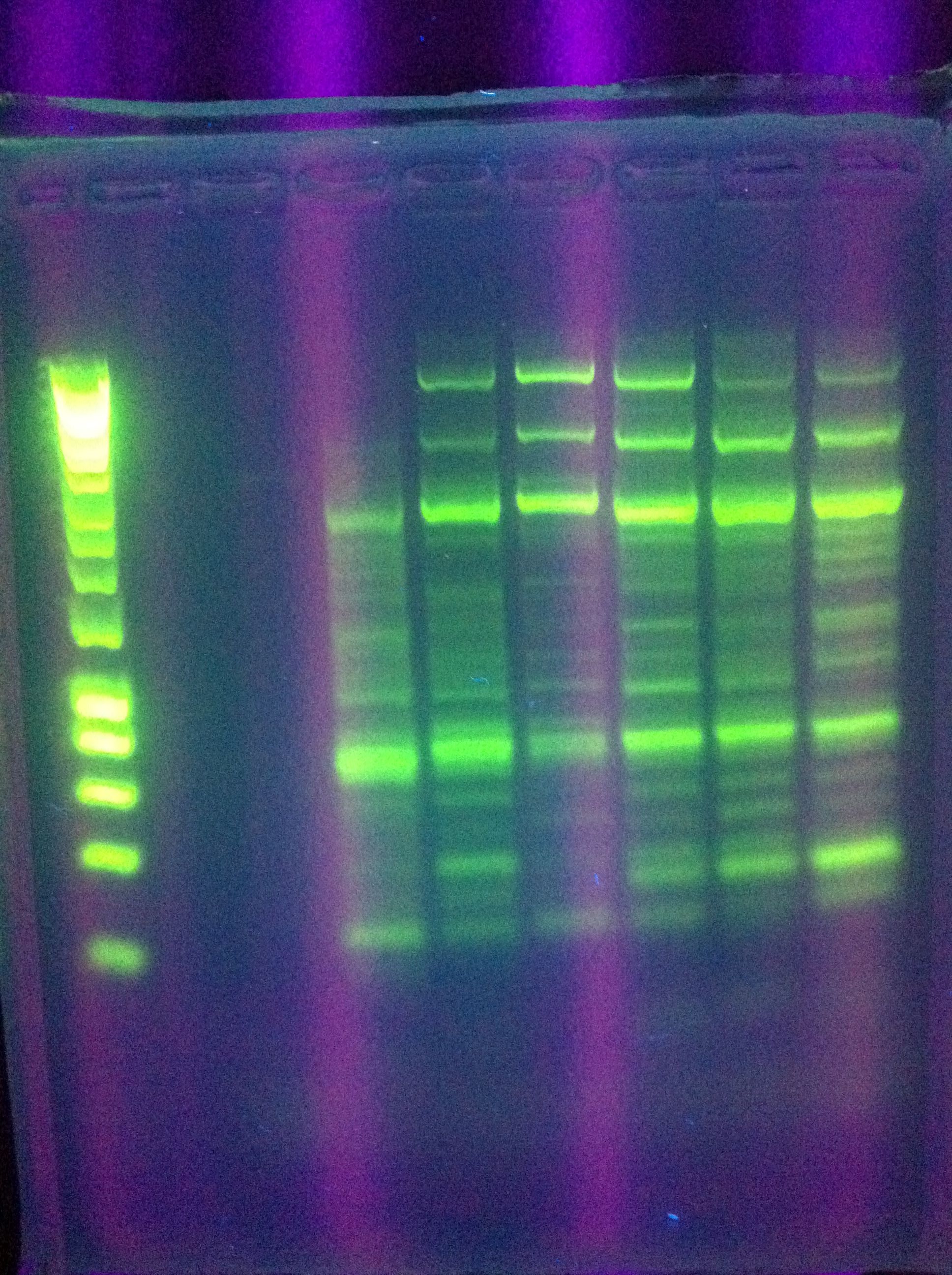
<br> [[Image:ASU_76_gel2.jpg|400px]] | <br> [[Image:ASU_76_gel2.jpg|400px]] | ||
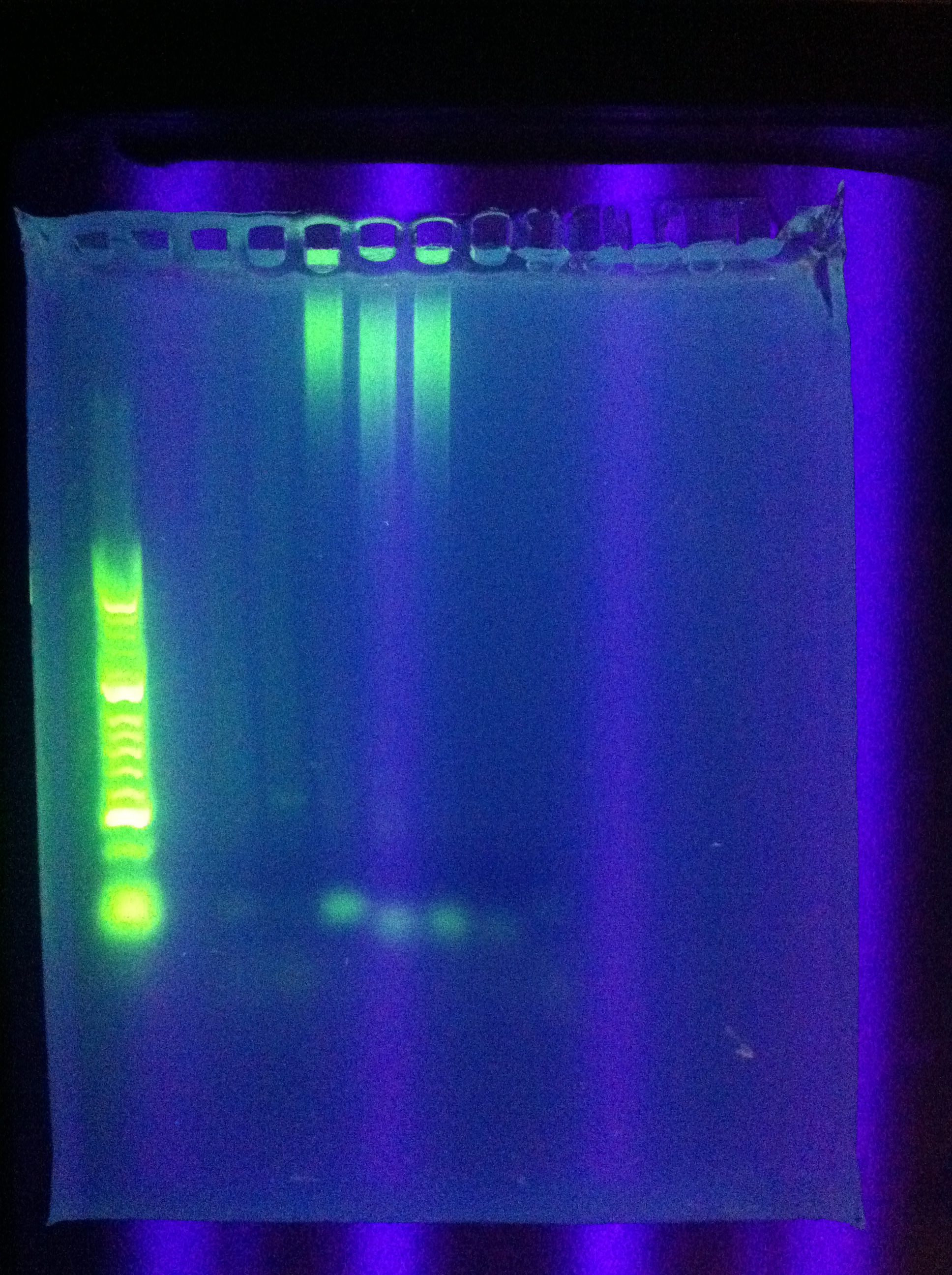
| - | :* | + | :* Ran Cas_d on gel, will visualize tomorrow morning |
| - | :* | + | :* Adjusted settings for cas_e_f, cas_3_r: 2 parallel runs on thermocycler |
::* touchdown to 70 using builtin touchdown | ::* touchdown to 70 using builtin touchdown | ||
::* constant 70 | ::* constant 70 | ||
| - | * | + | * Primer design - do we need new primers??? |
| - | :* melting temp mismatch? | + | :* Is there melting temp mismatch? |
| - | * lab supplies ordered (#4) | + | * More lab supplies ordered (#4) |
| - | * | + | * Made new amp plates |
== Thursday, July 7 == | == Thursday, July 7 == | ||
* Miniprep of: | * Miniprep of: | ||
| Line 80: | Line 80: | ||
:* DADA (ES, EX) | :* DADA (ES, EX) | ||
:* RARA (ES, EX) | :* RARA (ES, EX) | ||
| - | * Ligation (protocol from | + | * Ligation (protocol from OpenWetWare): |
:* 2x Seq1Seq1 | :* 2x Seq1Seq1 | ||
:* 2x DADA | :* 2x DADA | ||
| Line 92: | Line 92: | ||
<br> [[Image:ASU_77_plan2.jpg|400px]] | <br> [[Image:ASU_77_plan2.jpg|400px]] | ||
== Friday, July 8 == | == Friday, July 8 == | ||
| - | * promoter transformation: | + | * GFP promoter transformation: |
| - | :* | + | :* No success |
* GFP streak plate: | * GFP streak plate: | ||
| - | :* | + | :* Grew successfully |
| - | * DAx4, RAx4, | + | * DAx4, RAx4, Seq1 transformation: |
| - | :* | + | :* Successful, but very small colonies |
| - | * | + | * Received order from iGEM (pSB1A3 and other agar stab) |
| - | :* transformed | + | :* transformed pSB1A3 plasmid to make stock |
:* streak plated agar stab of J04430 | :* streak plated agar stab of J04430 | ||
== Sunday, July 10 == | == Sunday, July 10 == | ||
| - | * gel of array (1x, 4x): | + | * Ran gel of array (1x, 4x): |
| - | :* | + | :* Backbones visible for all |
| - | :* | + | :* No visible inserts in plasmid backbone |
* PCR of: | * PCR of: | ||
| - | :* | + | :* Homerun, EDC, AB, 3 |
| - | :* | + | :* None of these attempts work |
== Monday, July 11 == | == Monday, July 11 == | ||
| - | * | + | * Transformation results: |
| - | :* | + | :* pSB1A3: DNA contamination? Red colononies as well as normal, uncolored colonies growing on plates |
| - | ::* | + | ::* Possible contamination? We will need to sequence when we get biobrick primers |
| - | :* | + | :* Agar stab of part J04430: normal (glowing) |
| - | * made liquid cultures of: | + | * We made liquid cultures of: |
:* SEQ1, DA, RA (8x) in PSB1k3 | :* SEQ1, DA, RA (8x) in PSB1k3 | ||
:* PSB1A3 (pink, white colonies) | :* PSB1A3 (pink, white colonies) | ||
:* J04430 | :* J04430 | ||
| - | * | + | * Restriction: |
| - | :* | + | :* Restarting from 1x in PSB1K3 |
| - | :* | + | :* Ginkgo: |
| - | ::* | + | ::* Seq1: ES, XP |
| - | ::* | + | ::* DA: ES, XP |
| - | ::* | + | ::* RA: ES, XP |
| - | ::* | + | ::* E0840: EP to get PSB1A3 backbone |
| - | :* | + | :* Normal: |
| - | ::* | + | ::* Seq1: ES, EX |
| - | ::* | + | ::* DA: ES, EX |
| - | ::* | + | ::* RA: ES, EX |
* ligation: | * ligation: | ||
| - | :* 2x | + | :* 2x Seq1, DA, RA 2 ways: |
| - | ::* | + | ::* pSB1K3 |
| - | ::* | + | ::* pSB1A3 |
| - | * | + | * Transformation of 14 plates: |
| - | :* | + | :* New promoter (g18 on plate 1) in duplicate |
| - | :* | + | :* Ligation products in duplicate |
<br> [[Image:ASU_711_8xDA.jpg|400px]] | <br> [[Image:ASU_711_8xDA.jpg|400px]] | ||
<br> [[Image:ASU_711_gfp_construct_from_biobricks.jpg|400px]] | <br> [[Image:ASU_711_gfp_construct_from_biobricks.jpg|400px]] | ||
<br> [[Image:ASU_711_psb1a3_2.jpg|400px]] | <br> [[Image:ASU_711_psb1a3_2.jpg|400px]] | ||
| - | * | + | * Planning: |
| - | :* | + | :* Planned assembly of plasmid construct (cas genes, leader sequence, array) using pRSF-Duet plasmid |
| - | + | ||
<br> [[Image:ASU_711_plan2.jpg|400px]] | <br> [[Image:ASU_711_plan2.jpg|400px]] | ||
== Tuesday, July 12 == | == Tuesday, July 12 == | ||
| Line 179: | Line 178: | ||
<br> [[Image:ASU_712_progress.jpg|400px]] | <br> [[Image:ASU_712_progress.jpg|400px]] | ||
== Wednesday, July 13 == | == Wednesday, July 13 == | ||
| - | * | + | * We haven't been using alkaline phosphatase |
| - | :* | + | :* Haven't been preventing vector self ligation |
| - | ::* | + | ::* We have nothing! probably |
* ordered: | * ordered: | ||
| - | :* | + | :* AAP |
| - | * | + | * Answered safety questions |
| - | * | + | * Miniprepped promoter |
| - | * | + | * Still waiting on sequencing results |
== Thursday, July 14 == | == Thursday, July 14 == | ||
| - | * | + | * Miniprep: |
| - | :* | + | :* All constructs in puc57 |
| - | * | + | * Restriction: |
:* seq1: es, xp | :* seq1: es, xp | ||
:* da: es, xp | :* da: es, xp | ||
| Line 197: | Line 196: | ||
:* promoter: sp | :* promoter: sp | ||
:* e0840: xp | :* e0840: xp | ||
| - | * | + | * Ligation: |
:* da + da | :* da + da | ||
:* ra + ra | :* ra + ra | ||
:* seq1 + seq1 | :* seq1 + seq1 | ||
:* e0840 + promoter | :* e0840 + promoter | ||
| - | * | + | * Transformation: |
:* 2x da | :* 2x da | ||
:* 2x ra | :* 2x ra | ||
:* 2x seq1 | :* 2x seq1 | ||
| - | :* | + | :* E0840 + GFP promoter |
== Friday, July 15 == | == Friday, July 15 == | ||
| - | * | + | * Liquid cultures: |
| - | :* | + | :* LB + Kan: |
| - | ::* 2x | + | ::* 2x Seq1, DA, RA |
| - | * | + | * Transformation: |
| - | :* | + | :* Seq1, ra, da in puc57 (original from biobasic) |
| - | * | + | * Sequencing results: 3: |
| - | :* | + | :* Did not show inserts we want |
| - | :* piece of puc57, | + | :* A piece of puc57, E. Coli genome was sequenced |
| - | ::* contamination? | + | ::* Possible contamination? |
| - | :* | + | :* Use of AAP will prevent this in the future |
| - | * | + | * Fixed and submitted IDT leader sequence order |
| - | * | + | * Restriction/ligation of: |
| - | :* | + | :* E0840, GFP promoter |
:* normal (SP, XP): | :* normal (SP, XP): | ||
| - | ::* used AAP on both | + | ::* used AAP on both E0840 (XP) and promoter (SP) |
::* EP vector (kan) was added that should not have been added | ::* EP vector (kan) was added that should not have been added | ||
| - | ::* | + | ::* Was subsequently transformed onto kan plate, should have been amp |
| - | :* | + | :* Ginkgo (ES, XP, EP): |
| - | ::* | + | ::* Used AAP on psb1k3 vector (EP) |
| - | ::* | + | ::* Transformed and grown on kan plates |
| - | * | + | * Primers arrived! |
* PCR | * PCR | ||
| - | :* | + | :* CasABCDE |
| - | ::* | + | ::* Template concentration gradient (225 ng/uL, 112.5 ng/uL, 56 ng/uL) |
| - | ::* | + | ::* For each concentration, mgcl2 gradient (null, 1, 2, 4, 8) |
| - | :* | + | :* Cas3 |
| - | ::* | + | ::* Template concentration gradient (225 ng/uL, 112.5 ng/uL, 56 ng/uL) |
| - | ::* | + | ::* For each concentration, mgcl2 gradient (null, 1, 2, 4, 8) |
== Saturday, July 16 == | == Saturday, July 16 == | ||
| - | * | + | * Check transformants |
:* GFP construct (e0840, promoter) did not grow 3: | :* GFP construct (e0840, promoter) did not grow 3: | ||
:* 2x seq1, da, ra in psb1k3 | :* 2x seq1, da, ra in psb1k3 | ||
| - | * | + | * Liquid cultures |
:* Seq1, DA, RA in puc57, LB+Amp | :* Seq1, DA, RA in puc57, LB+Amp | ||
| - | :* | + | :* Original k12, LB |
| - | * | + | * Miniprep |
:* 2x seq1, da, ra in psb1k3 | :* 2x seq1, da, ra in psb1k3 | ||
| - | :* | + | :* Kylie will nanodrop this afternoon |
| - | * | + | * Glycerol stock |
:* 2x seq1, da, ra in psb1k3 | :* 2x seq1, da, ra in psb1k3 | ||
| - | :* | + | :* Threw away glycerol stocks of old 8x, 4x, 2x |
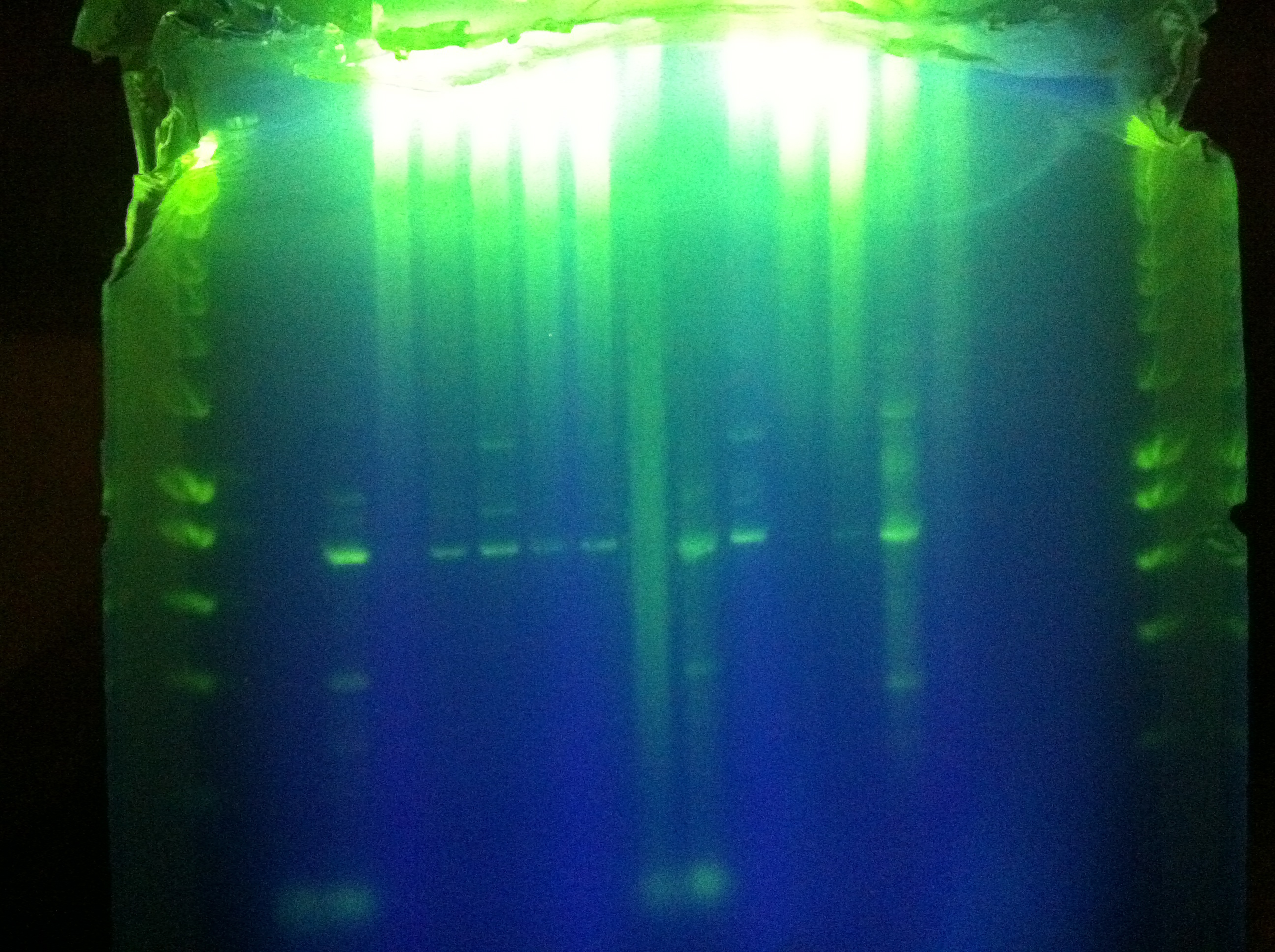
| - | * | + | * Gel electrophoresis |
| - | :* | + | :* CasABCDE |
| - | ::* | + | ::* Small gel, absolutely nothing visible |
| - | :* | + | :* Cas3 |
| - | ::* | + | ::* Big gel, also absolutely nothing 3: |
| - | :* | + | :* CMR |
| - | ::* SUCCESS | + | ::* SUCCESS - CMR is the thermocycler favorite |
<br> [[Image:ASU_716_cmr_success.jpg|400px]] | <br> [[Image:ASU_716_cmr_success.jpg|400px]] | ||
| - | ::* | + | ::* Extracted by Ethan |
| - | ::* | + | ::* This means our polymerase is fine, probably the template k12 that was wack |
| - | * | + | * Potential issues: |
| - | :* | + | :* Template DNA (which is why we are doing a new liquid culture of k12 for new genome prep) |
| - | :* | + | :* Polymerase problems? |
| - | :* | + | :* Something w/ master mix? |
:* Trinette the PCR machine didn't like the train of thermocycles running on her all day | :* Trinette the PCR machine didn't like the train of thermocycles running on her all day | ||
| + | ::*Choo Choo! | ||
:* PCR gnomes (DNA trafficking) | :* PCR gnomes (DNA trafficking) | ||
* PCR: | * PCR: | ||
| - | :* | + | :* CMR (using new primers) |
:* 220ng/ul null, 1, 2, 4, 8, 10 | :* 220ng/ul null, 1, 2, 4, 8, 10 | ||
:* 181ng/ul 1, 2, 4, 10 | :* 181ng/ul 1, 2, 4, 10 | ||
== Sunday, July 17 == | == Sunday, July 17 == | ||
| - | * | + | * Did a genome extraction of K-12 |
| - | * | + | * Miniprep: |
| - | :* | + | :* Seq1, ra, da in puc57 |
| - | :* | + | :* Very low concentrations likely due to inclusion of precipitate -- be careful w/ this step! |
| - | * | + | * Restriction: |
:* promoter: sp + aap | :* promoter: sp + aap | ||
:* e0840: xp + aap (accident) | :* e0840: xp + aap (accident) | ||
| Line 281: | Line 281: | ||
:* 2x da: es xp | :* 2x da: es xp | ||
:* psb1a3: ep + aap | :* psb1a3: ep + aap | ||
| - | * | + | * Ligation: |
:* promoter + e0840 | :* promoter + e0840 | ||
:* 2x seq1 -> 4x | :* 2x seq1 -> 4x | ||
:* 2x ra -> 4x | :* 2x ra -> 4x | ||
:* 2x da -> 4x | :* 2x da -> 4x | ||
| - | * | + | * Transformation: |
| - | :* | + | :* Using CCMB80 cells (old) |
::* old cells may not be competent / alive | ::* old cells may not be competent / alive | ||
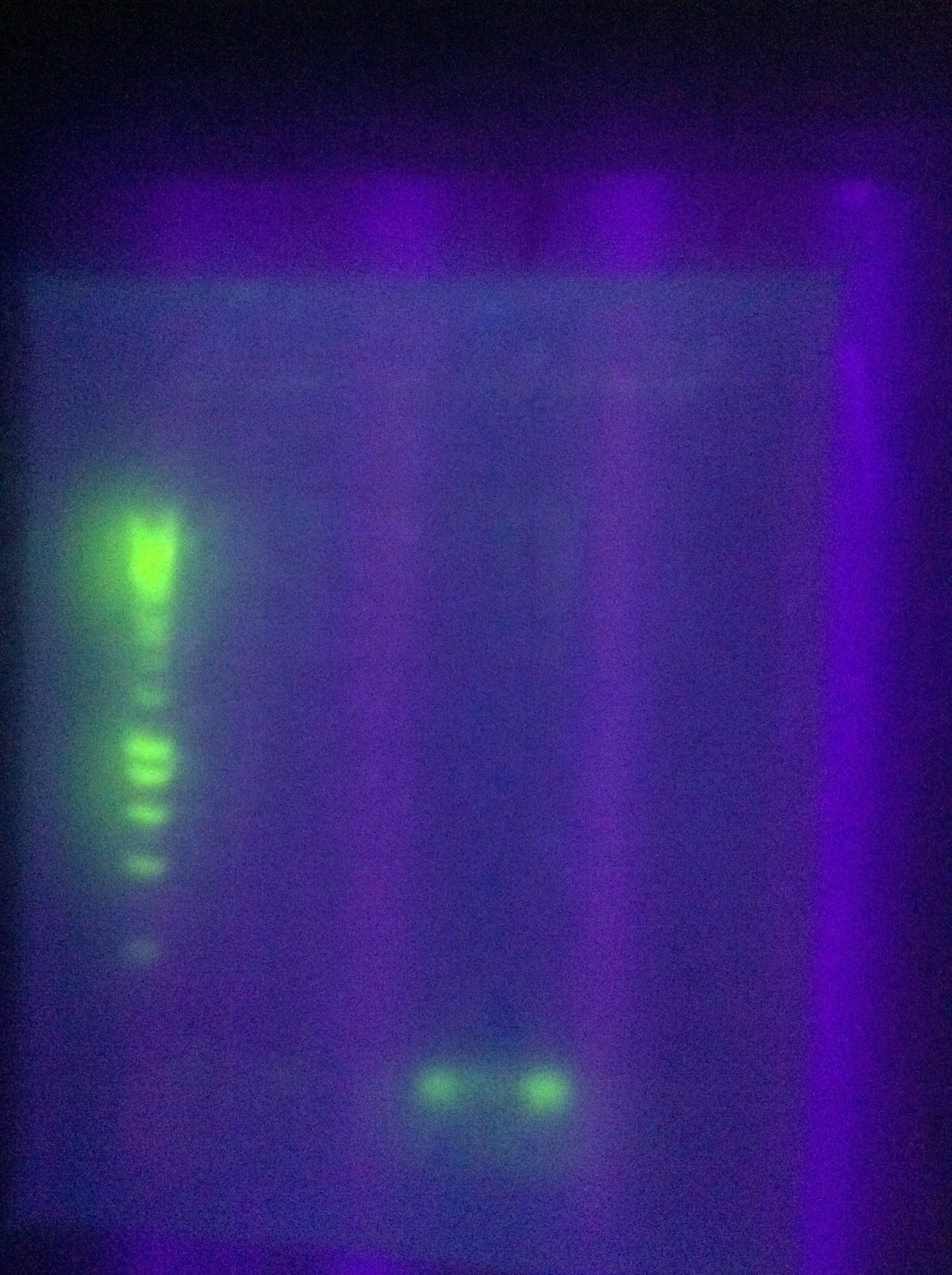
* PCR: | * PCR: | ||
| Line 301: | Line 301: | ||
<br> [[Image:ASU_717_cas_gel.jpg|400px]] | <br> [[Image:ASU_717_cas_gel.jpg|400px]] | ||
== Monday, July 18 == | == Monday, July 18 == | ||
| - | * | + | * Made new TSS cells |
| - | * | + | * Met with Catherine, got some good resources for modeling |
| - | :* | + | :* She will be less busy in August and can help us further |
* PCR: | * PCR: | ||
| - | :* | + | :* CasABCDE using a lower starting temp by 5 degrees, still touchdown |
| - | :* | + | :* Gel results: no bands at all |
* RLT: | * RLT: | ||
| - | :* | + | :* Traditional GFP construct |
| - | ::* | + | ::* GFP promoter (SP + AAP) |
::* E0840 (XP + gel extract) | ::* E0840 (XP + gel extract) | ||
| - | :* | + | :* Ginkgo GFP construct |
| - | ::* | + | ::* GFP promoter (ES) |
| - | ::* | + | ::* E0840 (XP) |
::* psb1k3 (EP + aap) | ::* psb1k3 (EP + aap) | ||
:* 4x constructs (ligation) | :* 4x constructs (ligation) | ||
| Line 322: | Line 322: | ||
* submitted sequencing: | * submitted sequencing: | ||
:* 2x: da, ra, seq1 | :* 2x: da, ra, seq1 | ||
| - | :* | + | :* CMR (f, r) |
| - | * Nanodropped 1x in puc57 miniprep by | + | * Nanodropped 1x in puc57 miniprep by Madeline |
| - | :* | + | :* Horrible results! |
| - | :* | + | :* Nisarg redid this miniprep today, will nanodrop tomorrow |
::* ((update: much better!)) | ::* ((update: much better!)) | ||
| - | * | + | * Made TSS cells w/ old BL21 DE3 |
| - | :* | + | :* We have asked Jon for fresh cells to make better comp cells |
== Tuesday, July 19 == | == Tuesday, July 19 == | ||
| - | * | + | * Transformation results: |
:* 4x Seq1 successful | :* 4x Seq1 successful | ||
| - | ::* | + | ::* However, we know this isn't actually 4x Seq1 |
:* 4x DA, RA unsuccessful | :* 4x DA, RA unsuccessful | ||
:* GFP construct unsuccessful | :* GFP construct unsuccessful | ||
| - | * | + | * Sequencing results: |
| - | :* | + | :* CMR: good =3 |
| - | :* | + | :* All else: nothing that we need 3: |
| - | ::* | + | ::* Just vector sequences |
| - | * | + | * New sequencing order: |
| - | :* | + | :* Putative RFP |
| - | :* | + | :* Original biobasic synthesis products in puc57 (with new puc57 primers) |
::* da, ra, seq1 | ::* da, ra, seq1 | ||
| - | :* | + | :* Our 1x transformation of biobasic synthesis products in puc57 (with new puc57 primers) |
::* 1xda, 1xra, 1xseq1 | ::* 1xda, 1xra, 1xseq1 | ||
| - | :* | + | :* Genomic DNA from mg1655 (k-12) to see if they can pull out cas gene sequences that we can't |
::* abcde f/r and cas3 f/r | ::* abcde f/r and cas3 f/r | ||
* PCR: | * PCR: | ||
| - | :* | + | :* Block A: |
| - | ::* | + | ::* Settings we used to get faint bands |
| - | ::* | + | ::* CasABCDE |
| - | ::* | + | ::* Touchdown: 70, -0.2 / cycle -> 63 |
| - | :* | + | :* Block B: |
| - | ::* | + | ::* Cas3 |
| - | ::* | + | ::* Gradient with 3 rows from 72 to 55 |
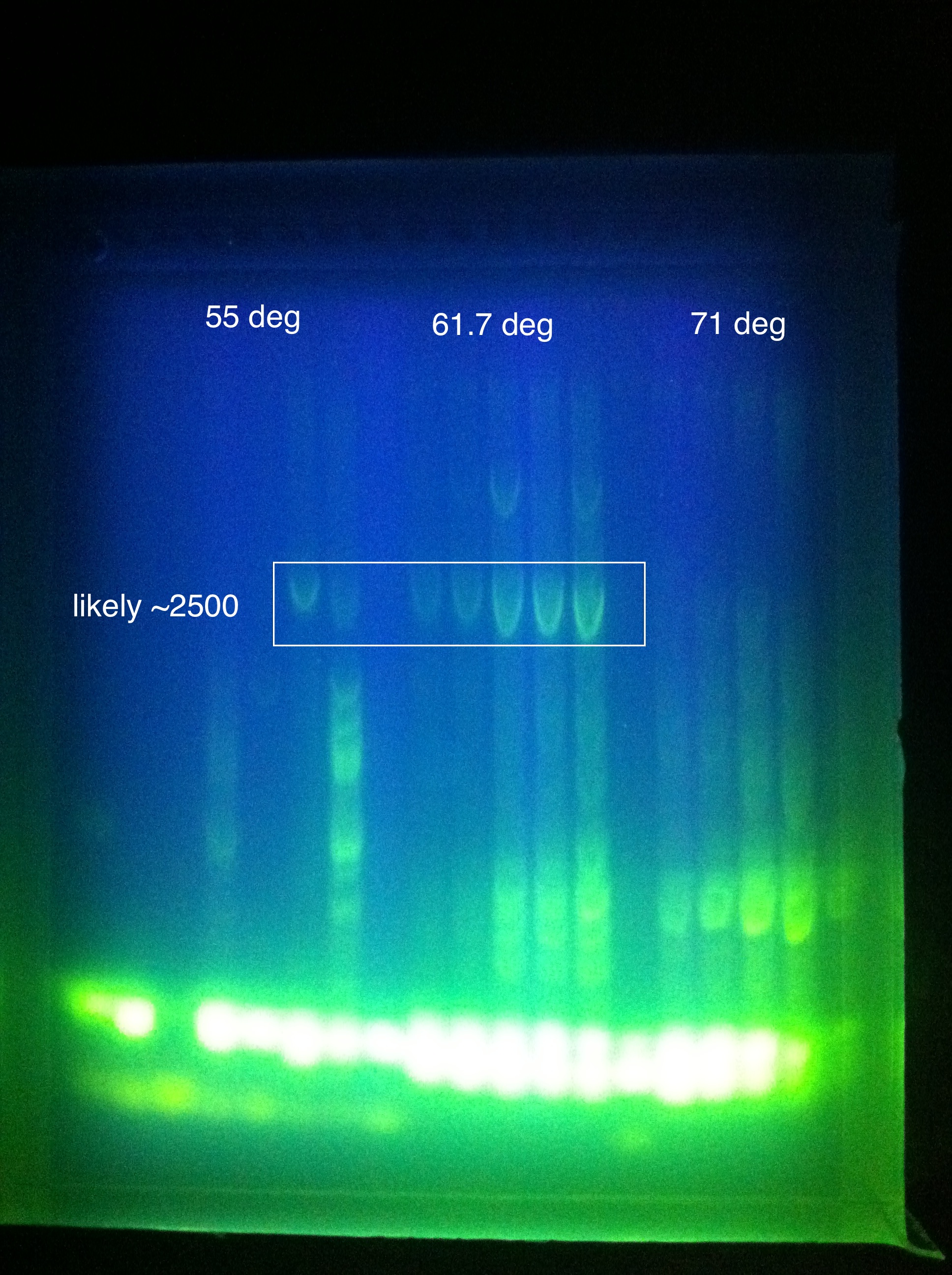
* Late night gel results: (Madeline and Joseph) | * Late night gel results: (Madeline and Joseph) | ||
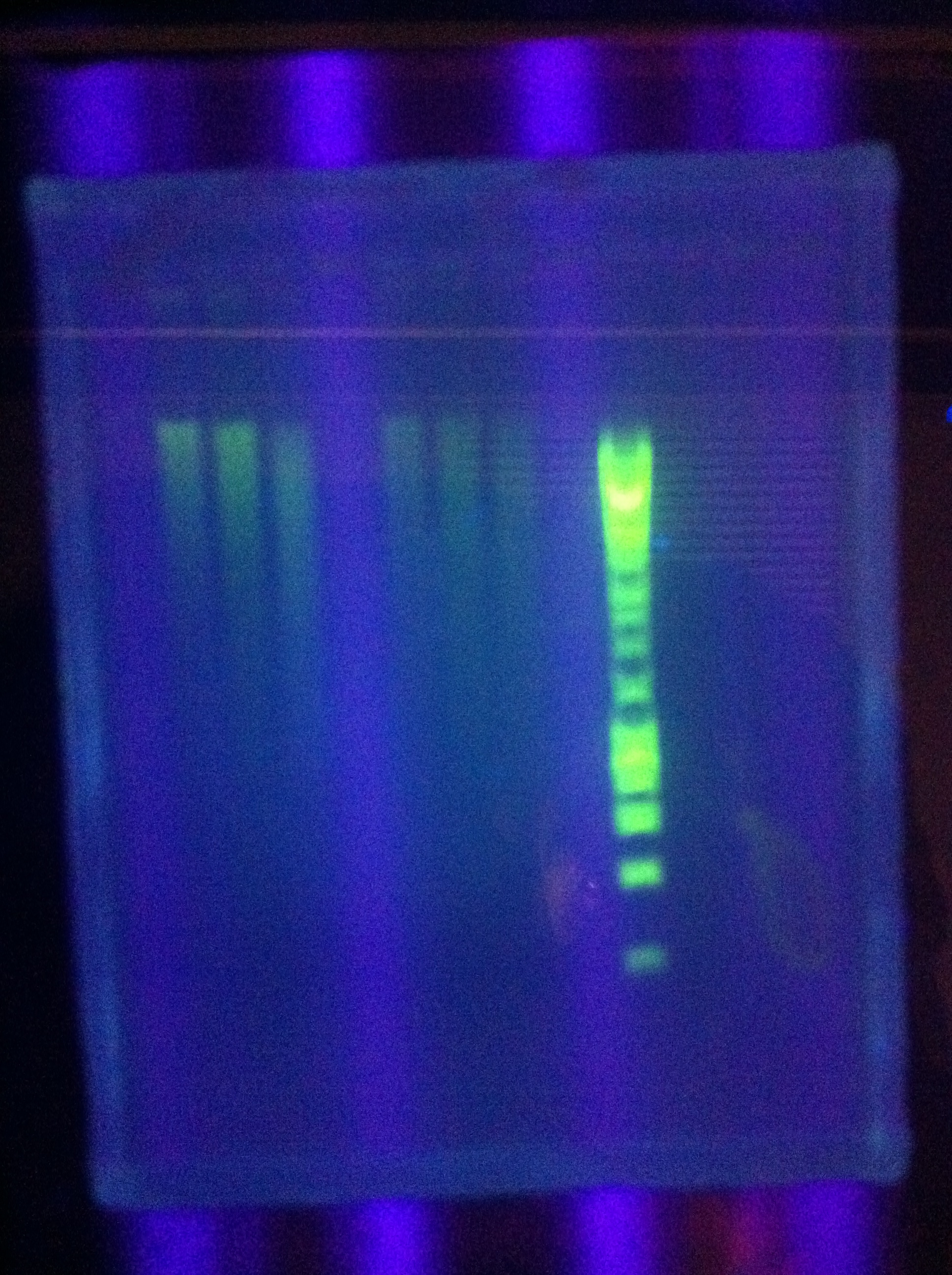
<br> [[Image:ASU_719_casABCDE.jpg|400px]] | <br> [[Image:ASU_719_casABCDE.jpg|400px]] | ||
| Line 362: | Line 362: | ||
::* So we THINK we got the desired band in the "midrange" temperature, which corresponds to "third up" row and relatively high magnesium chloride concentration | ::* So we THINK we got the desired band in the "midrange" temperature, which corresponds to "third up" row and relatively high magnesium chloride concentration | ||
::* SO we should do another PCR at approximately slightly above 60 somewhere | ::* SO we should do another PCR at approximately slightly above 60 somewhere | ||
| - | ::* | + | ::* Suggestion: gradient between 65 and 60 |
| - | ::* | + | ::* Also: what does it mean that it is curved? |
::* Also: what is the brightness at the bottom? happened in both gels... | ::* Also: what is the brightness at the bottom? happened in both gels... | ||
| - | * RLT: | + | * Restriction, Ligation and Transformation (RLT): |
| - | * | + | * Tried to gel E0840 cut w/ XP to get higher concentration of insert for ligation |
| - | ::* | + | ::* No bands at all 3: |
| - | :* | + | :* Instead, ligated / transformed purified GFP insert from yesterday |
:* @ 3ng/uL with approximately 40 uL, therefore ~120 ng of GFP insert (E0840) | :* @ 3ng/uL with approximately 40 uL, therefore ~120 ng of GFP insert (E0840) | ||
::* w/ 5 uL of vector (promoter cut at SP and w/ AAP) | ::* w/ 5 uL of vector (promoter cut at SP and w/ AAP) | ||
| Line 375: | Line 375: | ||
::* total: 52.5 uL reaction | ::* total: 52.5 uL reaction | ||
* Other news: | * Other news: | ||
| - | :* | + | :* Got new bacterial strains from Jon |
| - | :* | + | :* Submitted Barrett reimbursement forms for gas, food |
| - | :* | + | :* Met w/ Alana Labelle about safety, who told us to complete a "Preliminary Hazard Assessment" form |
| - | :* | + | :* New primers for duet vector and puc57 arrived |
:* GOT ICE ACCESS!!! | :* GOT ICE ACCESS!!! | ||
:* Kylie/Keith battle has begun | :* Kylie/Keith battle has begun | ||
| - | :* Xiao is proud of us | + | :* Xiao is proud of us :3 |
* To do: | * To do: | ||
| - | :* | + | :* Email iGEM to see what's up w/ Indianapolis accommodations |
| - | :* | + | :* Fill out form |
== Wednesday, July 20 == | == Wednesday, July 20 == | ||
| - | * | + | * Transformation results: |
:* 2 tiny colonies on one plate (GFP) | :* 2 tiny colonies on one plate (GFP) | ||
| - | :* | + | :* Made liquid culture (lb amp) |
| - | ::* | + | ::* Did not grow |
* PCR: | * PCR: | ||
:* ABCDE: starting: 71, -0.2/cycle -> 64 | :* ABCDE: starting: 71, -0.2/cycle -> 64 | ||
| - | :* | + | :* Cas3: another gradient using tighter range around best temp from yesterday |
::* 65.6 | ::* 65.6 | ||
::* 63.1 | ::* 63.1 | ||
::* 61.2 | ::* 61.2 | ||
::* 58.5 | ::* 58.5 | ||
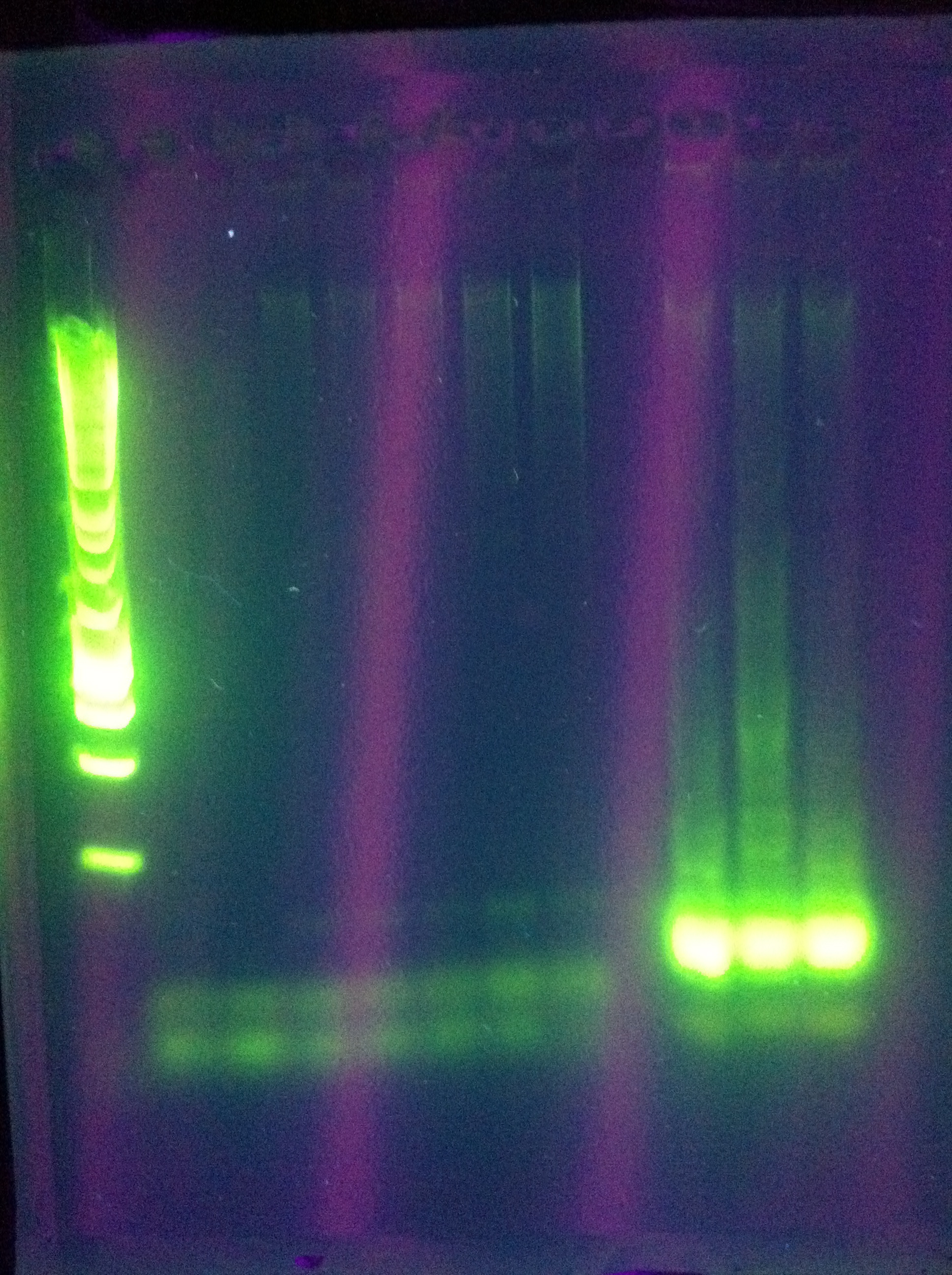
| - | :* | + | :* Results: |
<br> [[Image:ASU_720_CasABCDE.jpg|400px]] | <br> [[Image:ASU_720_CasABCDE.jpg|400px]] | ||
ABCDE: nothing usable | ABCDE: nothing usable | ||
<br> [[Image:ASU_720_Cas3.jpg|400px]] | <br> [[Image:ASU_720_Cas3.jpg|400px]] | ||
::* CAS3: small band at correct location | ::* CAS3: small band at correct location | ||
| - | :* | + | :* Need to order new primers with less dimerization potential! |
== Thursday, July 21 == | == Thursday, July 21 == | ||
* Heat shocked overnight RE digests (4 tubes of each for maximal gel results) | * Heat shocked overnight RE digests (4 tubes of each for maximal gel results) | ||
| Line 512: | Line 512: | ||
:* RAGE, Se1GE, PCR DA in A3 (Amp broth) | :* RAGE, Se1GE, PCR DA in A3 (Amp broth) | ||
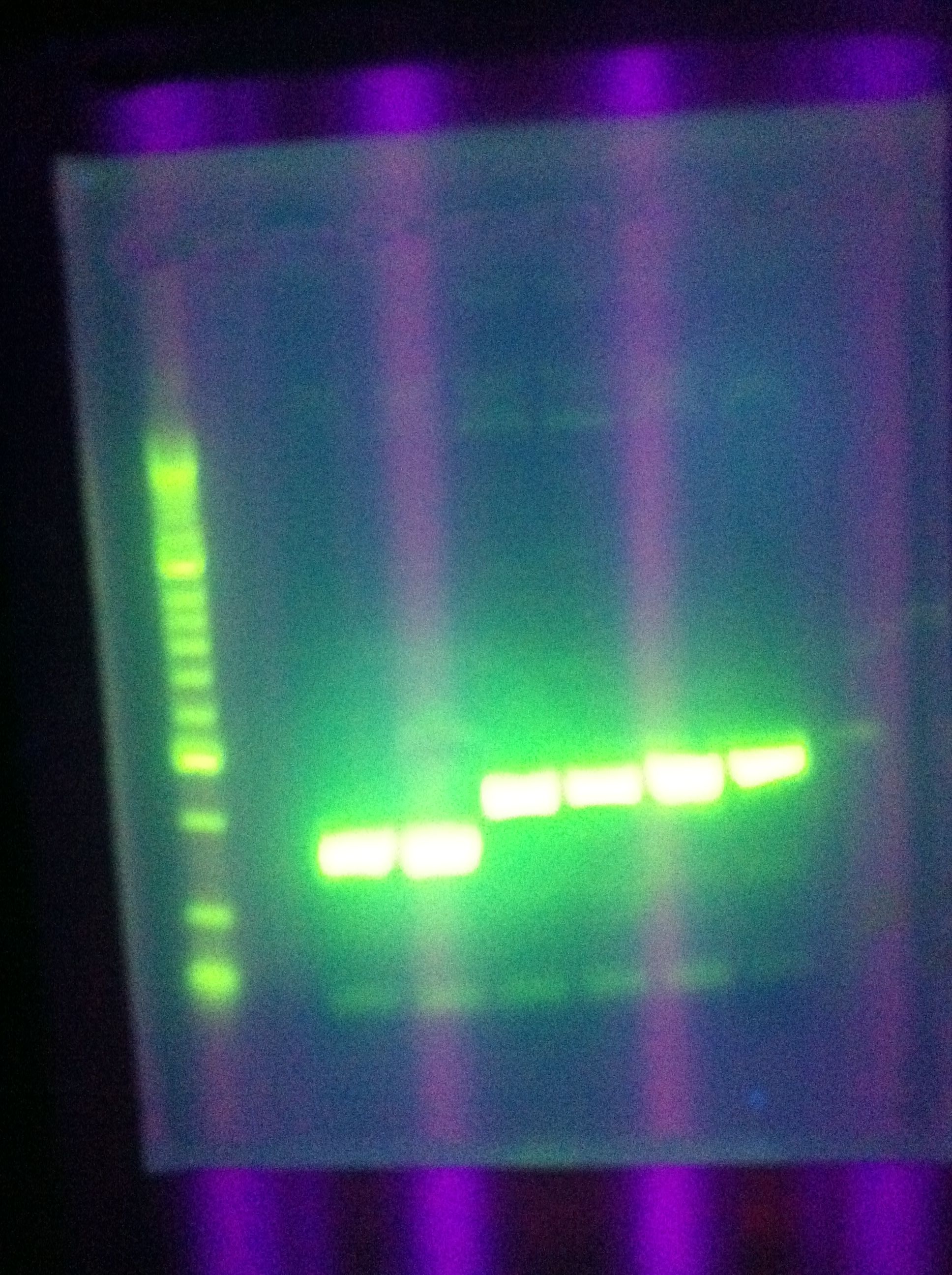
* Gel of PCR results | * Gel of PCR results | ||
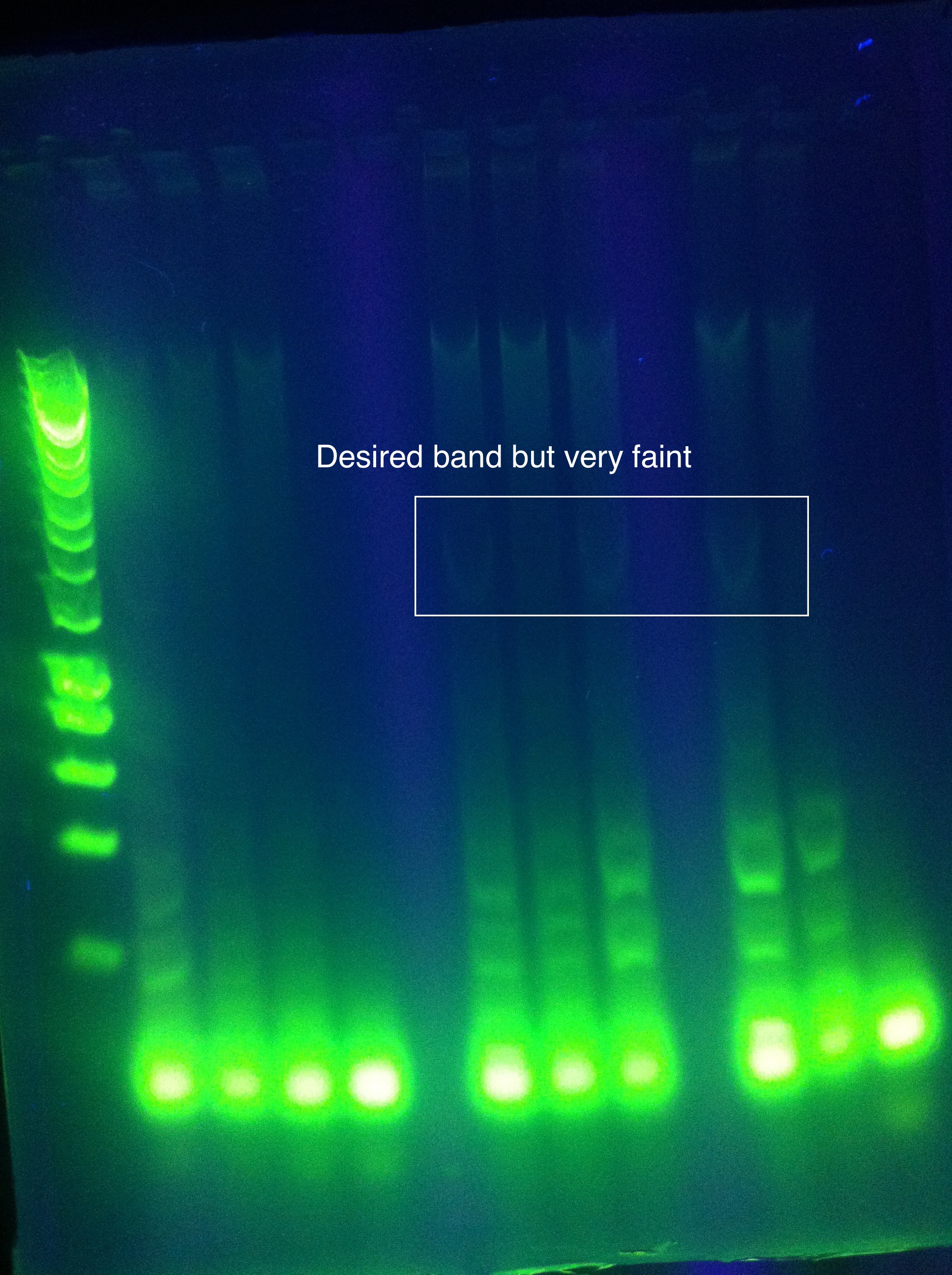
| - | <br> [[Image: | + | <br> [[Image:ASU_7_25_gel_abcde.jpeg|400px]] |
:* CasABCDE: not much visible, very faint and nonspecific | :* CasABCDE: not much visible, very faint and nonspecific | ||
::* Could be a result of changing the settings too much | ::* Could be a result of changing the settings too much | ||
| Line 578: | Line 578: | ||
:* Seq1, DA, RA 1x in psb1A3 (Forward/Reverse) | :* Seq1, DA, RA 1x in psb1A3 (Forward/Reverse) | ||
== Wednesday, July 27 == | == Wednesday, July 27 == | ||
| - | * | + | * Ligate 2x in psb1k3 |
| - | :* | + | :* Using overnight digests |
::* Seq1 in pSB1A3 (ES, XP) | ::* Seq1 in pSB1A3 (ES, XP) | ||
::* DA in pSB1A3 (ES, XP) | ::* DA in pSB1A3 (ES, XP) | ||
::* RA in pSB1A3 (ES, XP) | ::* RA in pSB1A3 (ES, XP) | ||
::* (EP) pSB1K3 from "DA in pSB1K3", which is not really DA | ::* (EP) pSB1K3 from "DA in pSB1K3", which is not really DA | ||
| - | * | + | * Transformation of this |
| - | * | + | * Begin duet construction: |
| - | :* | + | :* Transformed duet plasmid |
| - | * | + | * Streak plate RFP plates |
* PCR: | * PCR: | ||
| - | :* | + | :* CasABCDE with new and old primers and a temp gradient for both |
| - | :* | + | :* New: 64->69, three rows of 3 tubes |
| - | :* | + | :* Old: 63->66, three rows of 3 tubes |
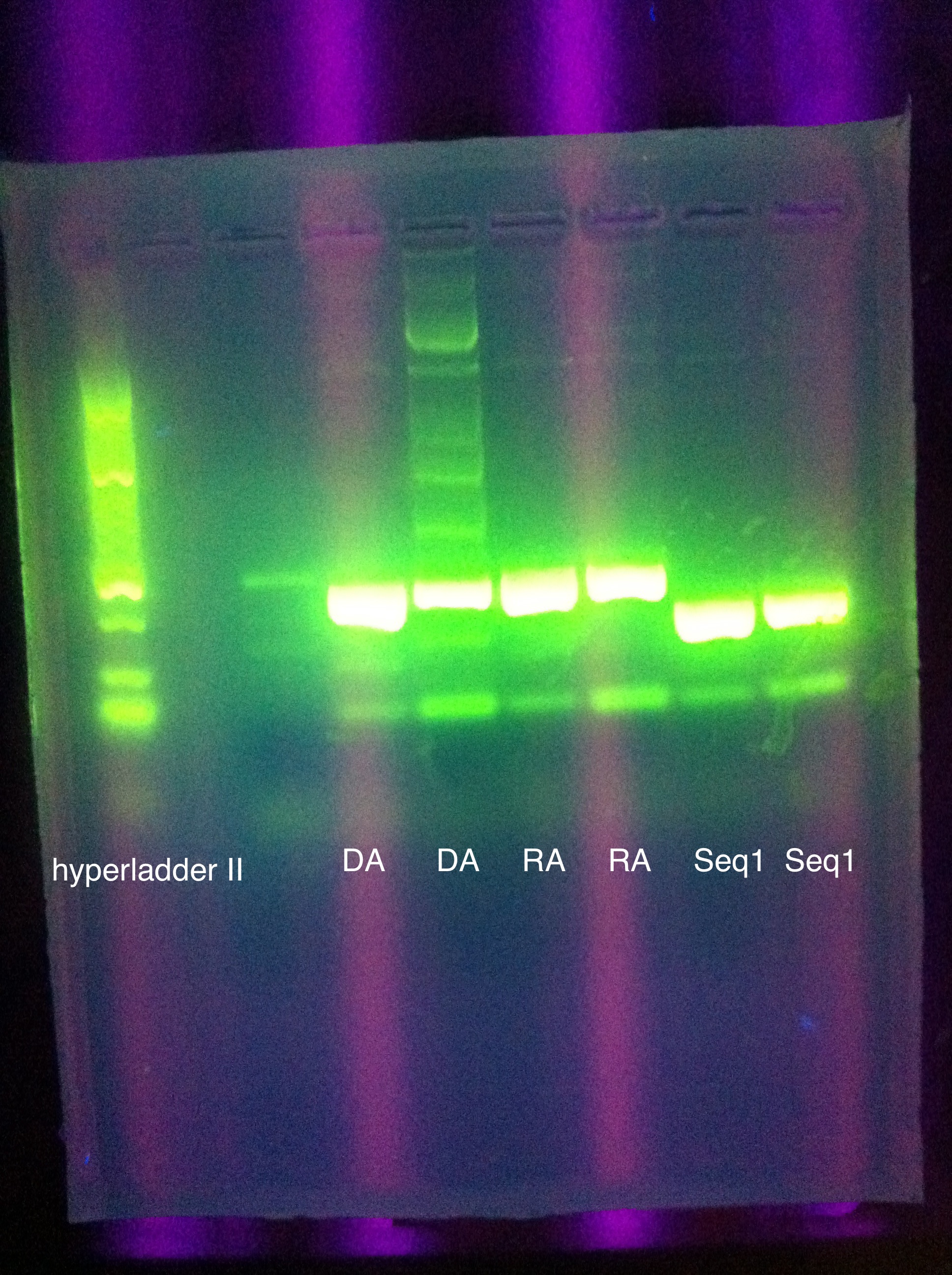
* Gel results: | * Gel results: | ||
<br> [[Image:ASU_727_IMG_3952.jpg|400px]] | <br> [[Image:ASU_727_IMG_3952.jpg|400px]] | ||
| - | :* | + | :* New -- no good |
| - | :* | + | :* Old -- mostly blank with some dimerization |
| - | * | + | * Made more LB + kan, amp, no antibiotic plates |
| - | * | + | * Talked about CMR things |
| - | :* | + | :* We can buy P. furiosus DNA at 2 ug for $300 |
== Thursday, July 28 == | == Thursday, July 28 == | ||
| - | * | + | * Plates from yesterday: |
| - | :* | + | :* Negative control with amp: growing |
:* RFP streak plates: correct | :* RFP streak plates: correct | ||
| - | :* | + | :* Duet vector: correct |
:* 2x ra, da, seq1: did not grow | :* 2x ra, da, seq1: did not grow | ||
* Gel of restrictions from yesterday | * Gel of restrictions from yesterday | ||
| Line 619: | Line 619: | ||
::* 2:10 elongation | ::* 2:10 elongation | ||
* Gel results: | * Gel results: | ||
| - | :* | + | :* No good! ran these at night, all on one gel, and only one lane had anything at all and even then it was one large section of nonspecific amplification |
| - | * | + | * Meeting with grad students: |
| - | :* | + | :* Homology between casA and human immune proteins (which ones?) |
| - | ::* | + | ::* Could be an evolutionary thing |
| - | :* | + | :* Look into this could also be |
| - | ::* | + | ::* Conserved functional domains |
== Friday, July 29 == | == Friday, July 29 == | ||
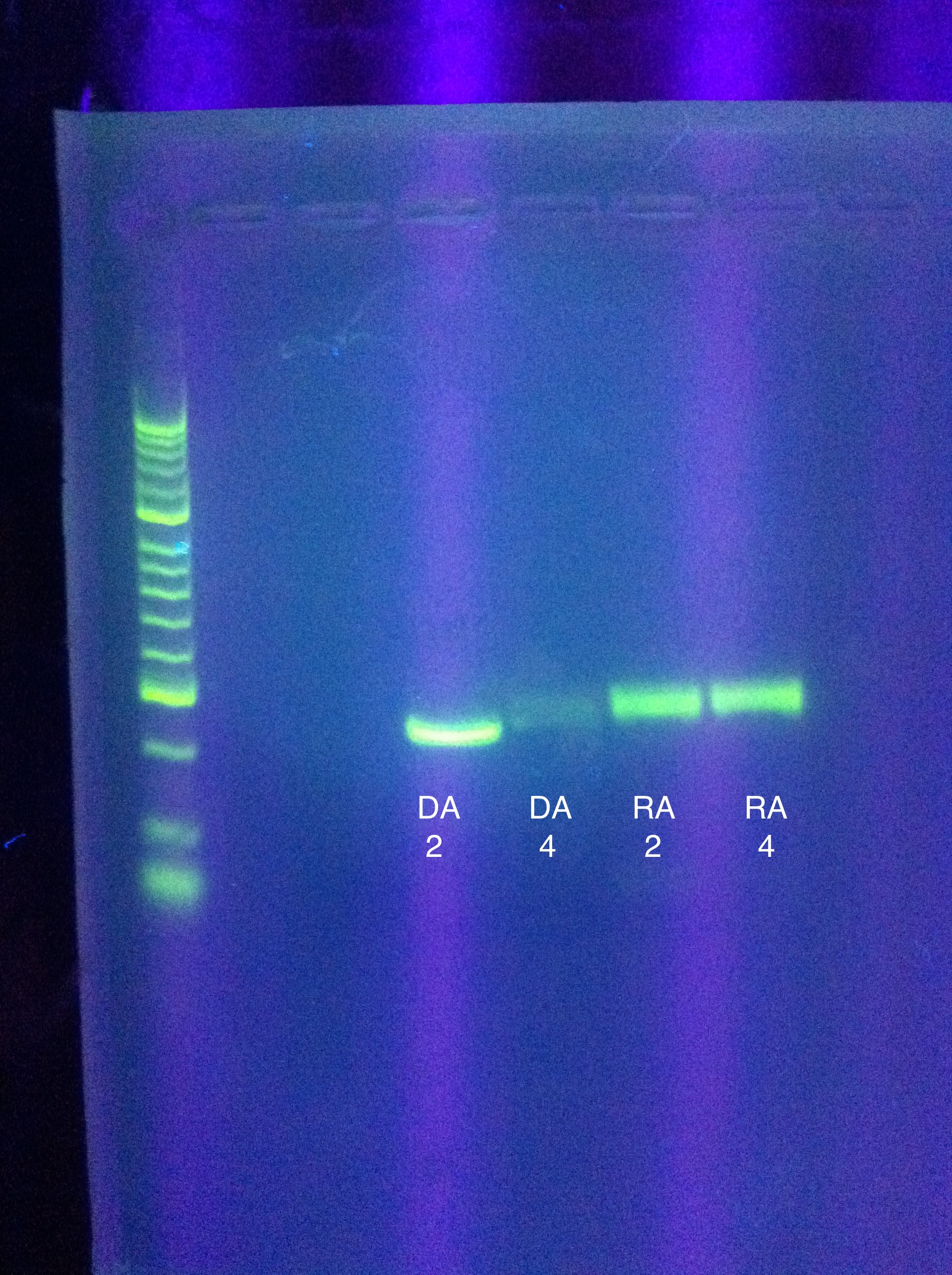
* Transformation results from yesterday | * Transformation results from yesterday | ||
| Line 642: | Line 642: | ||
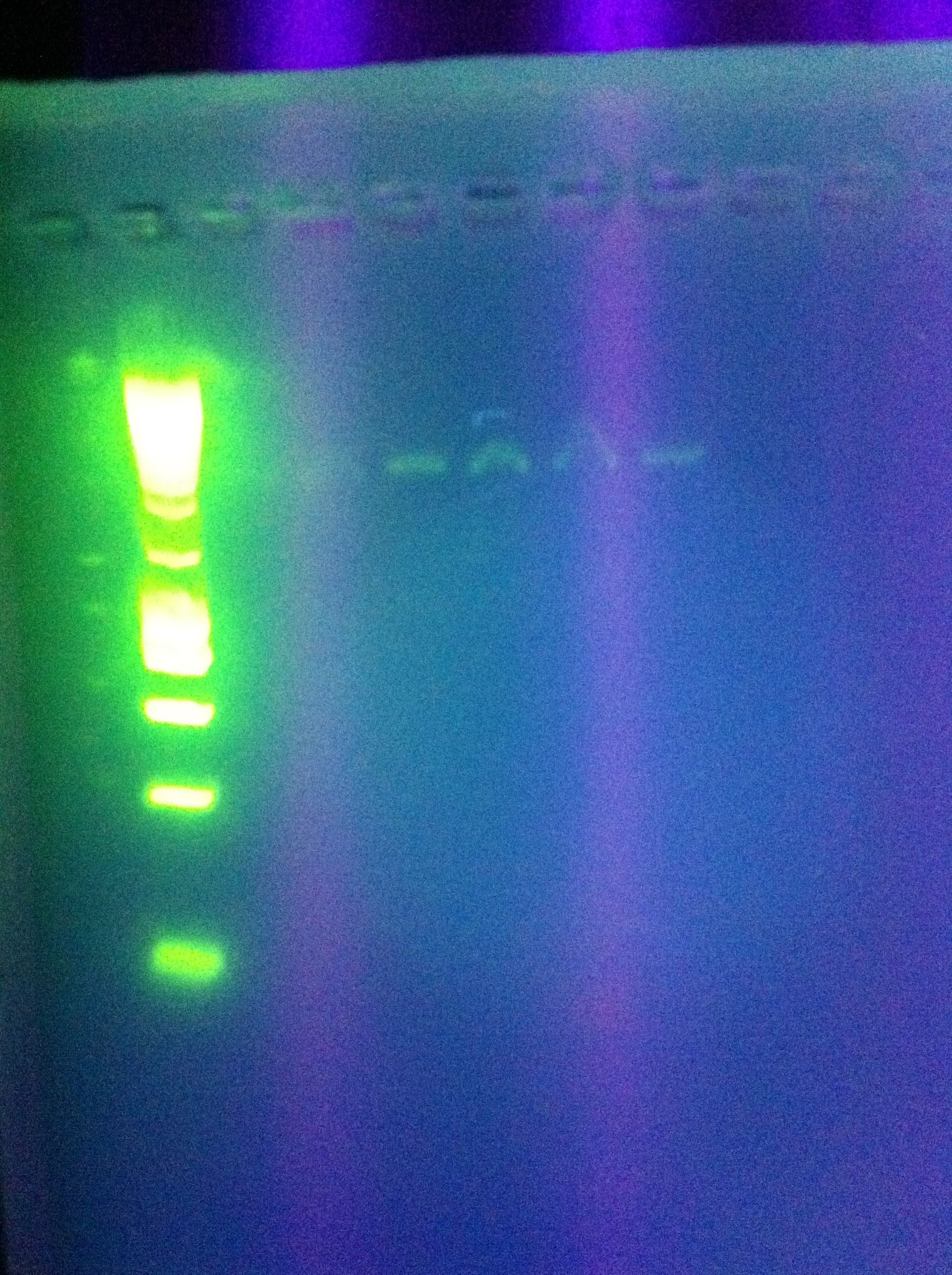
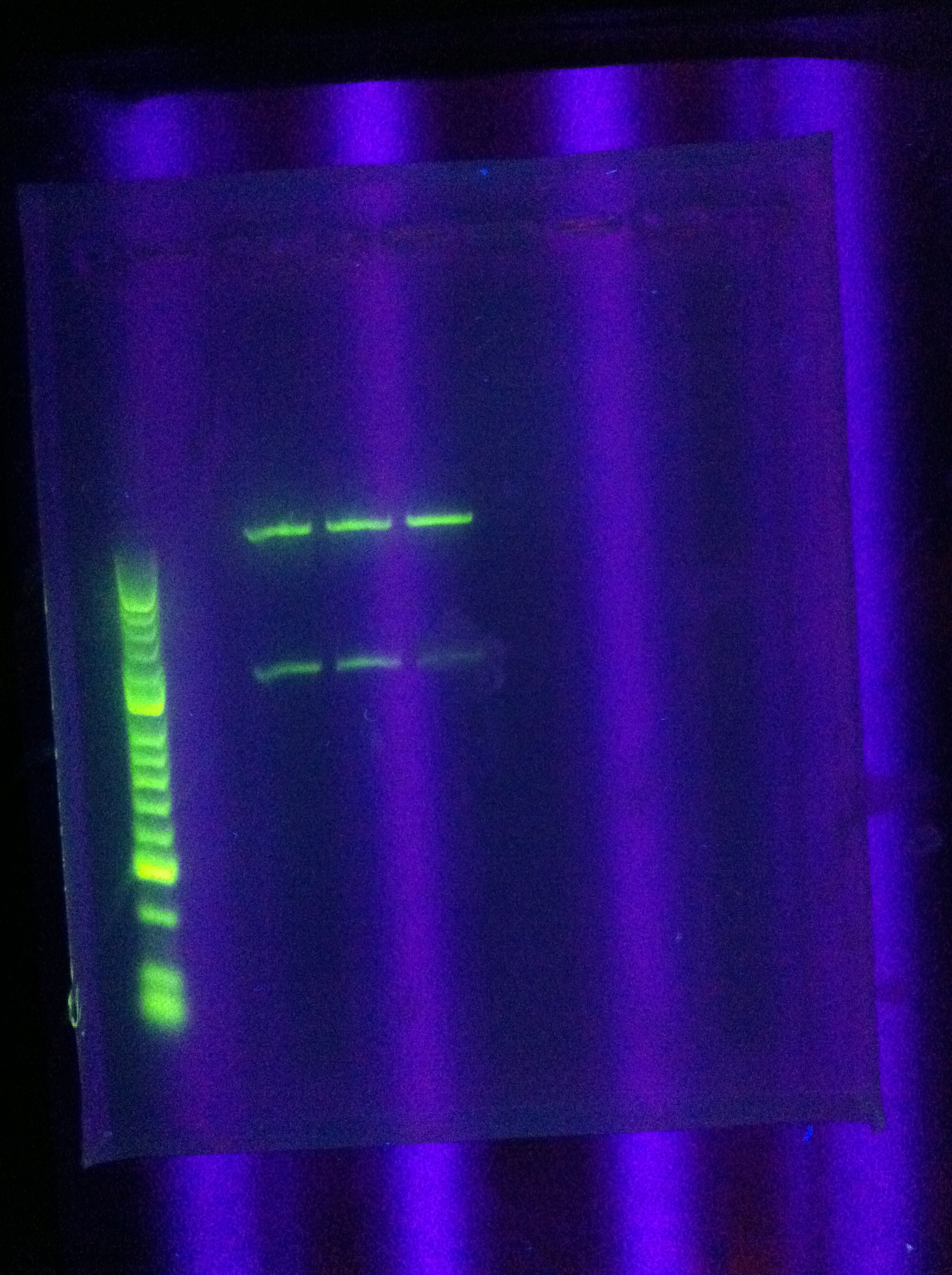
* Night Results | * Night Results | ||
:* Gels: No good. Nothing. "Dimer Central" | :* Gels: No good. Nothing. "Dimer Central" | ||
| + | <br> [[Image:ASU_7_29_gel.jpg|400px]] | ||
:* Miniprep of 1xDA | :* Miniprep of 1xDA | ||
:* Made SOC mini-stocks | :* Made SOC mini-stocks | ||
| Line 652: | Line 653: | ||
== Sunday, July 31 == | == Sunday, July 31 == | ||
* Gel Results of "Touchup" | * Gel Results of "Touchup" | ||
| - | :* | + | :* Worthless |
* Check plates | * Check plates | ||
:* No transformants but perhaps a few small ones | :* No transformants but perhaps a few small ones | ||
Latest revision as of 02:54, 29 September 2011
|
|
Friday, July 1
Saturday, July 2
Tuesday, July 5
Wednesday, July 6
Thursday, July 7
Friday, July 8
Sunday, July 10
Monday, July 11
Tuesday, July 12
Wednesday, July 13
Thursday, July 14
Friday, July 15
Saturday, July 16
Sunday, July 17
Monday, July 18
Tuesday, July 19
Wednesday, July 20
Thursday, July 21
Friday, July 22
Saturday, July 23
Sunday, July 24
Monday, July 25
Tuesday, July 26
Wednesday, July 27
Thursday, July 28
Friday, July 29
Saturday, July 30
Sunday, July 31
|
 "
"