Team:UCL London/Manufacturing/PurityYieldCost/ProcessDetails
From 2011.igem.org
(Difference between revisions)
| Line 15: | Line 15: | ||
''Right:'' Flow Sheet of Novel DNA Vaccine Manufacturing Process with E.coili | ''Right:'' Flow Sheet of Novel DNA Vaccine Manufacturing Process with E.coili | ||
| - | [[File:Ucl-content-Manufacturing-tables.jpg|center]] | + | [[File:Ucl-content-Manufacturing-tables.jpg|center]]<br /> |
| + | ''(Abbereviations: UF/DF, Ultrafiltration/Diafiltration; AEC, Anion Exchange Chromatography; SEC, Size Exclusion Chromatography.)'' | ||
</div> | </div> | ||
{{:Team:UCL_London/Template/Footer}} | {{:Team:UCL_London/Template/Footer}} | ||
Revision as of 22:15, 21 September 2011
Process Details
Hence, based on the market research,
The amount of product needed to meet the market requirement
= 1,000,000 treatments per yr × 0.005g per treatment = 5 kg per year
Assume: 20 batches per year
Final product yield per batch = 5 kg/20 = 0.25 kg
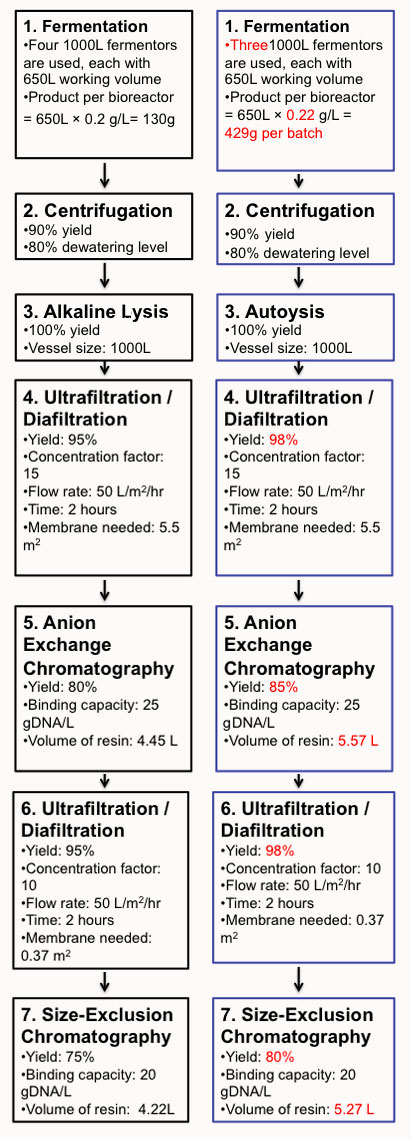
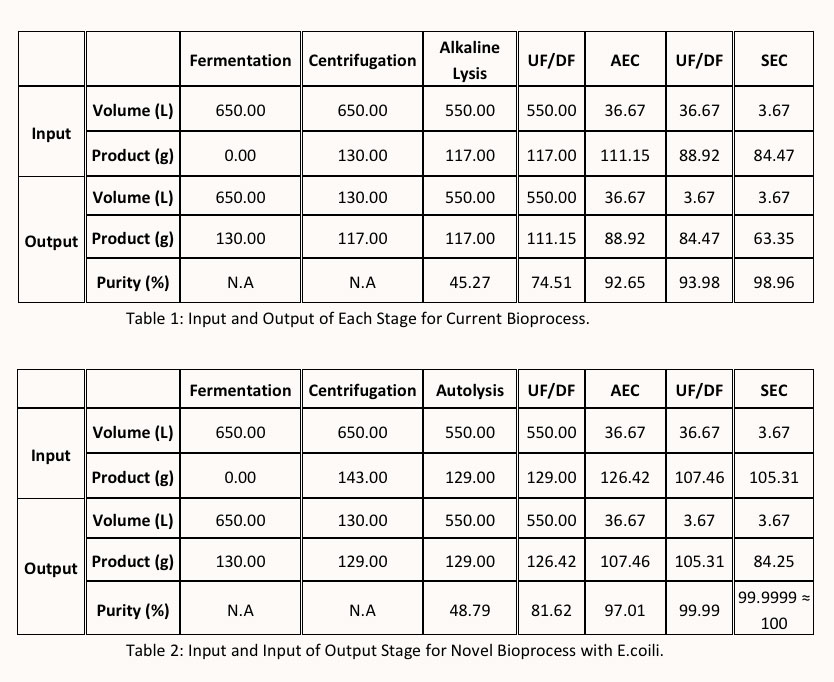
Figure 1. Comparison between the Current and Novel Bioprocesses.
Left: Flow Sheet of Current DNA Vaccine Manufacturing Process
Right: Flow Sheet of Novel DNA Vaccine Manufacturing Process with E.coili
(Abbereviations: UF/DF, Ultrafiltration/Diafiltration; AEC, Anion Exchange Chromatography; SEC, Size Exclusion Chromatography.)
 "
"