Team:Valencia/Pruebabea
From 2011.igem.org
| Line 268: | Line 268: | ||
<p>Here we show a glimpse of our results. You can see the observed effect produced by our colicins. | <p>Here we show a glimpse of our results. You can see the observed effect produced by our colicins. | ||
</p> | </p> | ||
| + | |||
| + | <center>><img src="https://static.igem.org/mediawiki/2011/6/63/Comopetent_lisys.jpg" width="400"/><img src="https://static.igem.org/mediawiki/2011/5/5b/ValenciaTeam2011_negativecontrols.JPG" width="400"/></center> | ||
| + | |||
Revision as of 18:25, 21 September 2011
Here we show a glimpse of our results. You can see the observed effect produced by our colicins.


To check the different colicin activities, we sow wild type Escherichia coli in different plates. After 10h, we added 100 µL of the different liquid colicin-producer cultures to each plate. Finally, 20h later we noticed a blue halo in the plates, indicating the death of wild type E. coli. The negative control plates did not present this coloring.
We want to study the different colicin and microcin properties regarding pH-dependence, minimum active concentration, time dynamics, etc.
In order to gather all this information we designed different experiments. Unfortunately, we are still lacking enough experimental results, so most of the statistical and mathematical models we have developed are not validated, yet. Even so, we can advance that the activity vs. concentration model follows a logistic growth model.
Antimicrobial peptides
Antimicrobial peptides (AMPs) are a diverse group of gene-encoded natural antibiotics found in all living organisms. In multicellular organisms, such as mammalians, defensins and cathelicidins are a principal component of innate immunity. In unicellular organisms, bacteriocins function to suppress competitor species. AMPs kill bacteria by disruption of membrane integrity and are less likely to induce resistance than common antibiotics, so they have attracted attention in different fields such as medicine and food safety.
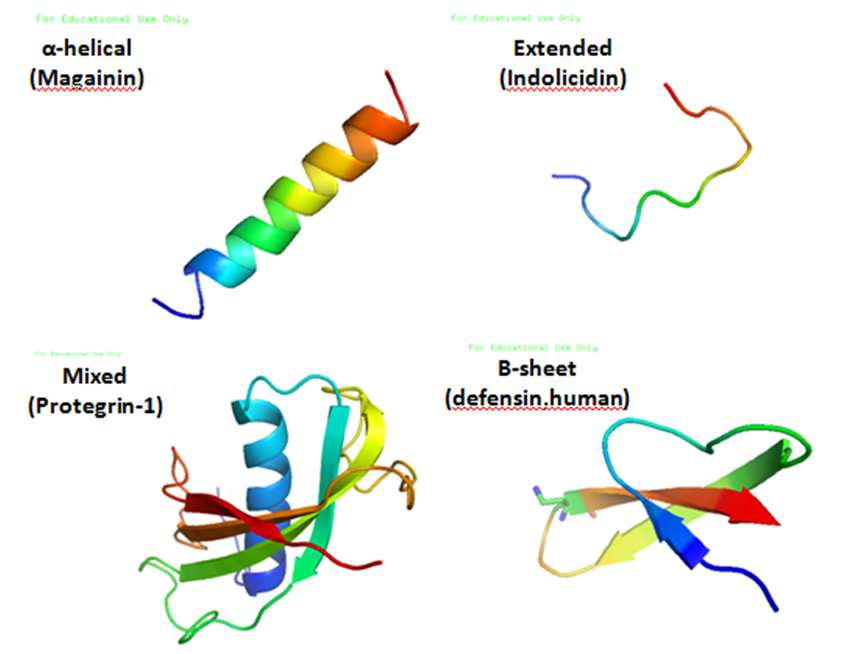
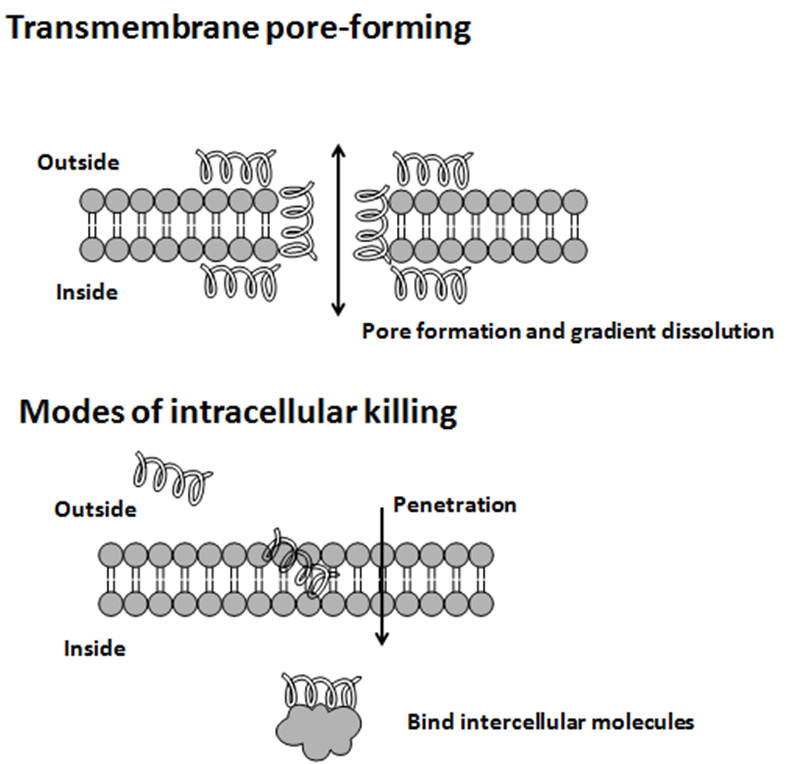
Bacteriocins
Bacteriocins are lethal to bacteria other than the producing strain. As a group, bacteriocins are heterogeneous and are classified according to their molecular weight. The activity spectrum of bacteriocins can be narrow and confined to inhibition of closely related species, or it can be relatively broad and include many different bacterial species. Bacteriocins are often considered more natural than common antibiotics because they are thought to have been present in many of the foods eaten since ancient times. This is one of the reasons why we have chosen bacteriocins over other antimicrobial peptides.
Microcins
Microcins are a peculiar class of gene-encoded low-molecular-mass antibacterial peptides secreted by enterobacteria. They contribute to the regulation of microbial competition within the intestinal microbiota. The genetic systems involved in microcin biosynthesis share a conserved organization. Similar to bacteriocins, microcins exert potent antibacterial activity directed against phylogenetically-related bacterial strains, with minimal inhibitory concentrations in the nanomolar range.
Usually, microcins exhibit "Trojan horse" mechanisms of antibacterial activity: some microcin structures are a mime of essential elements, permitting recognition by outer membrane receptors used for vital functions in bacteria and further translocation into the periplasmic space, other microcins are secreted as harmless molecules and further processed in susceptible bacteria to form the toxic entity. When inside target bacteria, microcins bind essential enzymes or interact with the inner membrane to form a bacterial killing structure.
Microcin C51
Microcin C51 is a nucleopeptide of 1.18 kDa molecular mass and the first described nucleopeptide antibiotic. It contains a heptapeptide with the N-terminal formylmethionine and C-terminal asparagine bound to AMP via an aliphatic chain. Microcine C51 is characterized by a broad spectrum of action. Expression in Escherichia coli is activated upon decelerated growth of cells during their transition to the stationary growth phase. This synthesis is controlled by four genes organized in an operon.
Our microcin strain has come from Severinov Laboratory at Rutgers State University (Severinov et al. Mol Microbiol. 2007)
Colicin G and Colicin H
Colicins are proteins produced by some strains of Escherichia coli that are lethal for related strains of E. coli. Colicins of strain CA46 (colicin G producer) and of strain CA58 (colicin H producer) should be the same, and indeed, the sensitivity patterns were nearly identical.
Only the triple mutant receptors fepA cir fiu was completely resistant to the colicins from these strains. (i) the production of colicin by colicinogenic E. coli cells is induced by SOS agents, as is seen with lysogenic phages, and is lethal for producing cells. (ii) the produced colicin is released into the medium late after synthesis (later shown not to be the case for all colicins). (iii) colicin kills sensitive cells according to single-hit kinetics. (iv) colicin is not active against the producing bacteria if there is presence of a specific antagonist protein called the immunity protein.
All colicins are organized into three domains, each corresponding to one step of colicin action:
- The N-terminal domain is involved in translocation through the membrane.
- The central domain is involved in binding to the receptor.
- The C-terminal domain contains the active part.
The sequence of the colicin G and colicin H determinants have been deposited under the accession numbers AJ515251 and AJ515252, respectively, in the EMBL/GenBank database.
- Escherichia coli microcin operon, strain CA46 (CA46 ColG)
- Escherichia coli microcin operon, strain CA58 (CA58 ColH)

Bibliography
Cascales E et al. Colicin Biology. Microbiol Mol Biol Rev. 2007 March; 71(1): 158–229.
Joerger RD. Alternatives to Antibiotics: Bacteriocins, Antimicrobial Peptides and Bacteriophages. 2003 Poultry Science 82:640–647
Patzer SI et al. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology (2003), 149, 2557–2570
Severinov K, Semenova E, Kazakov A, Kazakov T, Gelfand MS. Low-molecular-weight post-translationally modified microcins. Mol Microbiol. 2007 Sep;65(6):1380-94
 "
"

