Team:Valencia/Project1
From 2011.igem.org
| Line 1: | Line 1: | ||
| - | |||
{{Template:Valencia-0-3}} | {{Template:Valencia-0-3}} | ||
| - | + | ||
| + | <h1>Antimicrobial peptides</h1> | ||
Antimicrobial peptides (AMPs) are a diverse group of gene-encoded natural antibiotics found in all living organisms. In multicellular organisms, such as mammalians, defensins and cathelicidins are a principal component of innate immunity. In unicellular organisms, bacteriocins function to suppress competitor species. AMPs kill bacteria by disruption of membrane integrity and are less likely to induce resistance than common antibiotics, so they have attracted attention in different fields such as medicine and food safety. | Antimicrobial peptides (AMPs) are a diverse group of gene-encoded natural antibiotics found in all living organisms. In multicellular organisms, such as mammalians, defensins and cathelicidins are a principal component of innate immunity. In unicellular organisms, bacteriocins function to suppress competitor species. AMPs kill bacteria by disruption of membrane integrity and are less likely to induce resistance than common antibiotics, so they have attracted attention in different fields such as medicine and food safety. | ||
| Line 9: | Line 9: | ||
<a href="http://en.wikipedia.org/wiki/Antimicrobial_peptides"><img src="http://upload.wikimedia.org/wikipedia/commons/c/c0/Modes_of_action.png" alt="AMPs' Mode of action" title="AMPs' Mode of action" width="399" height="382"></a></center></html> | <a href="http://en.wikipedia.org/wiki/Antimicrobial_peptides"><img src="http://upload.wikimedia.org/wikipedia/commons/c/c0/Modes_of_action.png" alt="AMPs' Mode of action" title="AMPs' Mode of action" width="399" height="382"></a></center></html> | ||
| - | + | <h2>Bacteriocins</h2> | |
| - | + | <p>The bacteriocins are protein compounds produced by bacteria that inhibit or kill closed related species. As a group, bacteriocins are heterogeneous and are classified according to their molecular weight. The activity spectrum of bacteriocins can be narrow and confined to inhibition of closely related species, or it can be relatively broad and include many different bacterial species. Bacteriocins are often considered more natural than common antibiotics because they are thought to have been present in many of the foods eaten since ancient times. This is one of the reasons why we have chosen bacteriocins over other antimicrobial peptides. | |
| + | </p> | ||
| - | + | <h2>Colicins</h2> | |
| + | <h3>Colicin G and Colicin H</h3> | ||
| - | + | <p>Colicins are by far the best-characterized group of bacteriocins. The colicins are protein compounds produced by, and active against, <i>Escherichia coli</i> and others members of <i>Enterobacteriaceae</i> family. | |
| + | </p> | ||
| - | + | <p>At least 34 different colicins have been described and found to share an interesting number of features. The release of colicins is in itself not always lethal to the producing cell. The colicinogenic bacteria are specifically protected against the colicins they produce. In addition, colicin gene clusters are plasmid encoded. | |
| + | </p> | ||
| - | + | <p>Under conditions of stress a fraction of colicinogenic bacteria are induced to produce colicin proteins. The type of stress causing the inductive activity needs to be better characterized. The release of colicins is in itself not always lethal to the producing cell. In addition, colicin gene clusters are plasmid encoded. A number of different applications have been suggested for colicins due to their broad spectrum of action. | |
| - | + | </p> | |
| - | + | <p>Colicins kill sensitive bacteria in three main and distinct ways. The most frequent mechanism is the formation of ion channels in the plasma membrane (pore formation), resulting in membrane depolarization. The opening of the pore also induces a phosphate and sometimes K+ efflux, which leads to depletion of cytoplasmic ATP. | |
| + | </p> | ||
| - | + | <p>All colicins are organized into three domains, each corresponding to one step of colicin action | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | All colicins are organized into three domains, each corresponding to one step of colicin action | + | |
<ul> | <ul> | ||
<li>The '''N-terminal domain''' is involved in translocation through the membrane.</li> | <li>The '''N-terminal domain''' is involved in translocation through the membrane.</li> | ||
| Line 43: | Line 35: | ||
<li>The '''C-terminal domain''' contains the active part.</li> | <li>The '''C-terminal domain''' contains the active part.</li> | ||
</ul> | </ul> | ||
| + | </p> | ||
| - | The sequence of the | + | <p>The production of colicin by colicinogenic E. coli cells is induced by SOS agents. The produced colicin is released into the medium late after synthesis. And it kills sensitive cells according to single-hit kinetics, not being active against the producing bacteria if there is presence of a specific antagonist protein called the immunity protein, which is in our strains. |
| + | </p> | ||
| + | |||
| + | <p><i>Escherichia coli</i> strains CA46(pColG) and CA58(pColH) each apparently synthesized two generally similar bactericidal colicin proteins whose molecular weights were approximately 5 500 and 100 000. And indeed, the sensitivity patterns were nearly identical. They use the same receptor on sensitive cells, probably the outer membrane protein <b>Fiu</b>, as indicated by the partial resistance of <i>fiu</i> mutants of <i>Escherichia coli</i> to both active proteins. | ||
| + | </p> | ||
| + | |||
| + | <p>The sequence of the colicin G and colicin H determinants have been deposited under the accession numbers AJ515251 and AJ515252, respectively, in the EMBL/GenBank database. | ||
| + | </p> | ||
<ul> | <ul> | ||
<li>[http://www.ncbi.nlm.nih.gov/nuccore/AJ515251 Escherichia coli microcin operon, strain CA46 (CA46 ColG]</li> | <li>[http://www.ncbi.nlm.nih.gov/nuccore/AJ515251 Escherichia coli microcin operon, strain CA46 (CA46 ColG]</li> | ||
| Line 50: | Line 50: | ||
</ul> | </ul> | ||
| + | |||
| + | <h2>Microcins</h2> | ||
| + | |||
| + | <h3>Microcin C51 (MccC51)</h3> | ||
| + | [[File:Valeciateam_MccC51.gif|right|frame|Microcin C51]] | ||
| + | <p>Microcins are a peculiar class of gene-encoded low-molecular-mass antibacterial peptides secreted by Enterobacteria. They contribute to the regulation of microbial competition within the intestinal microbiota. The genetic systems involved in microcin biosynthesis share a conserved organization. Similar to bacteriocins, microcins exert potent antibacterial activity directed against phylogenetically-related bacterial strains, with minimal inhibitory concentrations in the nanomolar range. | ||
| + | </p> | ||
| + | |||
| + | <p>Usually, microcins exhibit "<i>Trojan horse</i>" mechanisms of antibacterial activity: some microcin structures are a mime of essential elements, permitting recognition by outer membrane receptors used for vital functions in bacteria and further translocation into the periplasmic space, other microcins are secreted as harmless molecules and further processed in susceptible bacteria to form the toxic entity. When inside target bacteria, microcins bind essential enzymes or interact with the inner membrane to form a bacterial killing structure. | ||
| + | </p> | ||
| + | |||
| + | <p>Microcin C51 is a members of class I posttranslationally modified microcins (heavily posttranslationally modified antibacterial peptides produced by <i>Enterobacteriaceae</i> with molecular weights of less than 5 kDa). It has atypical structure for ribosomally synthesized peptides and target essential molecular machines that are validated drug targets. | ||
| + | </p> | ||
| + | |||
| + | <p>Microcin C51 is a nucleopeptide of 1.18 kDa molecular mass and the first described nucleopeptide antibiotic. It contains a heptapeptide with the N-terminal formylmethionine and C-terminal asparagine bound to AMP via an aliphatic chain. Microcine C51 is characterized by a broad spectrum of action. | ||
| + | </p> | ||
| + | |||
| + | <p>A 5.7-kb fragment of the pC51 plasmid of natural strain of <i>Escherichia coli</i> carry the genes involved in MccC51 production, secretion, and self-immunity. Expression in <i>Escherichia coli</i> is activated upon decelerated growth of cells during their transition to the stationary growth phase. This synthesis is controlled by four genes organized in an operon. | ||
| + | </p> | ||
| + | |||
| + | <p>Our microcin strain has come from <i>Severinov Laboratory at Rutgers State University</i> (Severinov et al. Mol Microbiol. 2007). | ||
| + | </p> | ||
[[File:comparison of microcin gene clusters.PNG|center|600px]] | [[File:comparison of microcin gene clusters.PNG|center|600px]] | ||
Revision as of 15:52, 21 September 2011
Contents |
Antimicrobial peptides
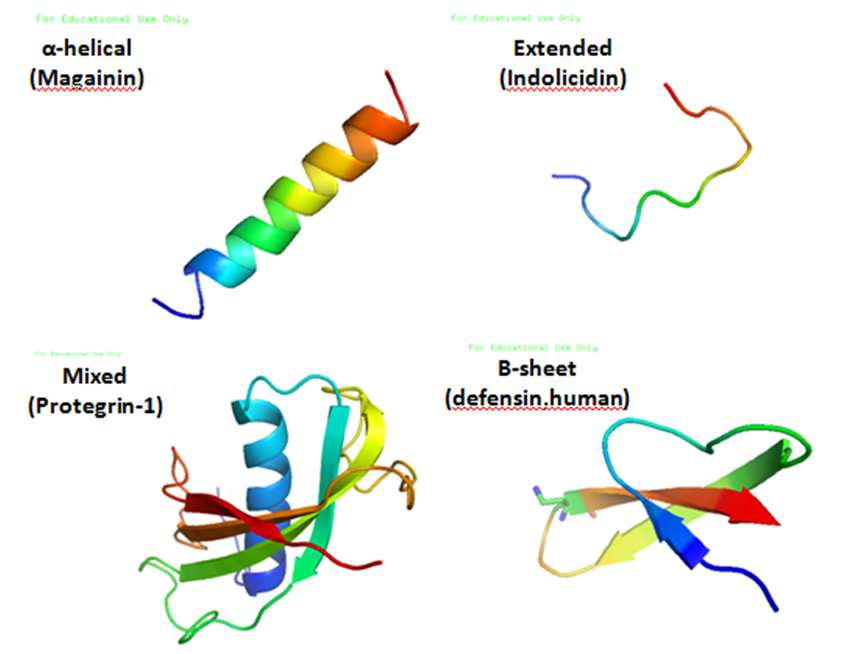
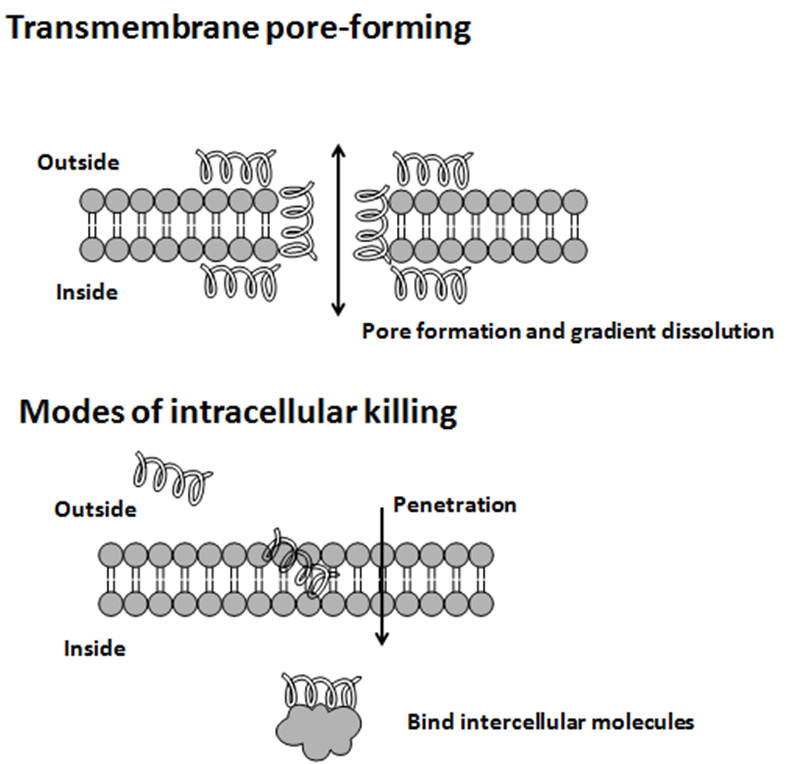
Antimicrobial peptides (AMPs) are a diverse group of gene-encoded natural antibiotics found in all living organisms. In multicellular organisms, such as mammalians, defensins and cathelicidins are a principal component of innate immunity. In unicellular organisms, bacteriocins function to suppress competitor species. AMPs kill bacteria by disruption of membrane integrity and are less likely to induce resistance than common antibiotics, so they have attracted attention in different fields such as medicine and food safety.
Bacteriocins
The bacteriocins are protein compounds produced by bacteria that inhibit or kill closed related species. As a group, bacteriocins are heterogeneous and are classified according to their molecular weight. The activity spectrum of bacteriocins can be narrow and confined to inhibition of closely related species, or it can be relatively broad and include many different bacterial species. Bacteriocins are often considered more natural than common antibiotics because they are thought to have been present in many of the foods eaten since ancient times. This is one of the reasons why we have chosen bacteriocins over other antimicrobial peptides.
Colicins
Colicin G and Colicin H
Colicins are by far the best-characterized group of bacteriocins. The colicins are protein compounds produced by, and active against, Escherichia coli and others members of Enterobacteriaceae family.
At least 34 different colicins have been described and found to share an interesting number of features. The release of colicins is in itself not always lethal to the producing cell. The colicinogenic bacteria are specifically protected against the colicins they produce. In addition, colicin gene clusters are plasmid encoded.
Under conditions of stress a fraction of colicinogenic bacteria are induced to produce colicin proteins. The type of stress causing the inductive activity needs to be better characterized. The release of colicins is in itself not always lethal to the producing cell. In addition, colicin gene clusters are plasmid encoded. A number of different applications have been suggested for colicins due to their broad spectrum of action.
Colicins kill sensitive bacteria in three main and distinct ways. The most frequent mechanism is the formation of ion channels in the plasma membrane (pore formation), resulting in membrane depolarization. The opening of the pore also induces a phosphate and sometimes K+ efflux, which leads to depletion of cytoplasmic ATP.
All colicins are organized into three domains, each corresponding to one step of colicin action
- The N-terminal domain is involved in translocation through the membrane.
- The central domain is involved in binding to the receptor.
- The C-terminal domain contains the active part.
The production of colicin by colicinogenic E. coli cells is induced by SOS agents. The produced colicin is released into the medium late after synthesis. And it kills sensitive cells according to single-hit kinetics, not being active against the producing bacteria if there is presence of a specific antagonist protein called the immunity protein, which is in our strains.
Escherichia coli strains CA46(pColG) and CA58(pColH) each apparently synthesized two generally similar bactericidal colicin proteins whose molecular weights were approximately 5 500 and 100 000. And indeed, the sensitivity patterns were nearly identical. They use the same receptor on sensitive cells, probably the outer membrane protein Fiu, as indicated by the partial resistance of fiu mutants of Escherichia coli to both active proteins.
The sequence of the colicin G and colicin H determinants have been deposited under the accession numbers AJ515251 and AJ515252, respectively, in the EMBL/GenBank database.
- [http://www.ncbi.nlm.nih.gov/nuccore/AJ515251 Escherichia coli microcin operon, strain CA46 (CA46 ColG]
- [http://www.ncbi.nlm.nih.gov/nuccore/AJ515252 Escherichia coli microcin operon, strain CA58 (CA58 ColH)]
Microcins
Microcin C51 (MccC51)
Microcins are a peculiar class of gene-encoded low-molecular-mass antibacterial peptides secreted by Enterobacteria. They contribute to the regulation of microbial competition within the intestinal microbiota. The genetic systems involved in microcin biosynthesis share a conserved organization. Similar to bacteriocins, microcins exert potent antibacterial activity directed against phylogenetically-related bacterial strains, with minimal inhibitory concentrations in the nanomolar range.
Usually, microcins exhibit "Trojan horse" mechanisms of antibacterial activity: some microcin structures are a mime of essential elements, permitting recognition by outer membrane receptors used for vital functions in bacteria and further translocation into the periplasmic space, other microcins are secreted as harmless molecules and further processed in susceptible bacteria to form the toxic entity. When inside target bacteria, microcins bind essential enzymes or interact with the inner membrane to form a bacterial killing structure.
Microcin C51 is a members of class I posttranslationally modified microcins (heavily posttranslationally modified antibacterial peptides produced by Enterobacteriaceae with molecular weights of less than 5 kDa). It has atypical structure for ribosomally synthesized peptides and target essential molecular machines that are validated drug targets.
Microcin C51 is a nucleopeptide of 1.18 kDa molecular mass and the first described nucleopeptide antibiotic. It contains a heptapeptide with the N-terminal formylmethionine and C-terminal asparagine bound to AMP via an aliphatic chain. Microcine C51 is characterized by a broad spectrum of action.
A 5.7-kb fragment of the pC51 plasmid of natural strain of Escherichia coli carry the genes involved in MccC51 production, secretion, and self-immunity. Expression in Escherichia coli is activated upon decelerated growth of cells during their transition to the stationary growth phase. This synthesis is controlled by four genes organized in an operon.
Our microcin strain has come from Severinov Laboratory at Rutgers State University (Severinov et al. Mol Microbiol. 2007).
Bibliography
Cascales E et al. Colicin Biology. Microbiol Mol Biol Rev. 2007 March; 71(1): 158–229.
Joerger RD. Alternatives to Antibiotics: Bacteriocins, Antimicrobial Peptides and Bacteriophages. 2003 Poultry Science 82:640–647
Patzer SI et al. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology (2003), 149, 2557–2570
Severinov K, Semenova E, Kazakov A, Kazakov T, Gelfand MS. Low-molecular-weight post-translationally modified microcins. Mol Microbiol. 2007 Sep;65(6):1380-94
 "
"
