Team:Paris Liliane Bettencourt/Notebook/2011/08/30/
From 2011.igem.org
(Created page with "== Cyrille == S24 + YFP-TetR: - Prepare the 9 miniprep - Replicate into glycerol - PCR colony was done File:CP3008_PCRColony.jpg YFP-TetR PstI-- 5re;oving the qdditionnal...") |
m (Camille) |
||
| (6 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | {{:Team:Paris_Bettencourt/tpl_test}} | ||
| + | |||
== Cyrille == | == Cyrille == | ||
S24 + YFP-TetR: | S24 + YFP-TetR: | ||
| - | + | * Prepare the 9 miniprep | |
| - | + | * Replicate into glycerol | |
| - | + | * PCR colony was done | |
[[File:CP3008_PCRColony.jpg]] | [[File:CP3008_PCRColony.jpg]] | ||
| Line 10: | Line 12: | ||
YFP-TetR PstI-- 5re;oving the qdditionnal PstI site | YFP-TetR PstI-- 5re;oving the qdditionnal PstI site | ||
| - | + | * 5 Miniprep prepared | |
Competent cell preparation of MH1 | Competent cell preparation of MH1 | ||
| + | |||
| + | |||
| + | == Axel == | ||
| + | ===extraction of ComS from 5 different colonies=== | ||
| + | same protocol as those from the 17th of august: | ||
| + | |||
| + | We are going to extract the ComS gene from the PY79 strain. Oligo has been designed for this purpose. | ||
| + | |||
| + | * Preparing the tube | ||
| + | |||
| + | Prepare 5 sterile tube with 20 µL of H2O for each colony | ||
| + | Pick the colony using a pipetman woth a sterile tube. The pipetor is set at 3 µL for mixing | ||
| + | |||
| + | * Dissolving the primers | ||
| + | |||
| + | Resuspend the primers adding 10µL of water by nmol of primer. Final concentration at 100 mM. | ||
| + | Add 1µL in 2 ml of water to get the 50 µM | ||
| + | |||
| + | * PCR mastermix | ||
| + | |||
| + | 60 µL HF phusion buffer | ||
| + | 6 µL dNTPs | ||
| + | 15 µL of colony mix | ||
| + | 6 µL Primer fwd at (50µM) | ||
| + | 6 µL Primer rev at (50µM) | ||
| + | 157 µL of H2O | ||
| + | |||
| + | Aliquot 49 µL in 5 PCR tubes | ||
| + | Add 1 µL of phusion in each tube. | ||
| + | |||
| + | * PCR temperature | ||
| + | |||
| + | 1 - 98°C - 15 min | ||
| + | 2 - 98°C - 30 sec | ||
| + | 3 - 70°C - gradient +-10 - 1 min | ||
| + | 4 - 55°C - 1 min | ||
| + | 5 - 72°C - 1 min | ||
| + | 6 - Repeat 2-5 39 times | ||
| + | 7 - 7°C forever | ||
| + | |||
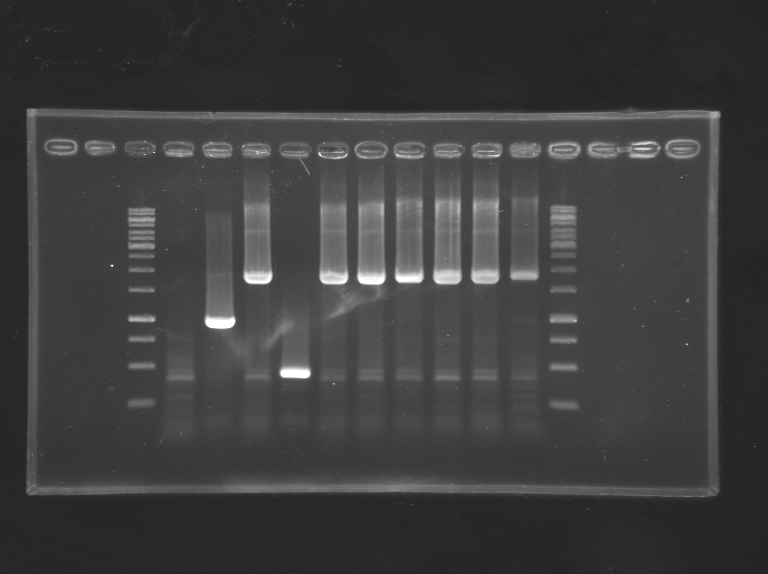
| + | result of the gel: (biobricked ComS is about 160bp) | ||
| + | ladder 100bp | ||
| + | [[File:ComS extraction.jpg|thumb|center|ladder - col1 - col2 - col3 - col4 - col5 - control no colony]] | ||
| + | |||
| + | |||
| + | ==Camille== | ||
| + | |||
| + | From the overnight culture I did minipreps, and obtained : | ||
| + | {| border="1" class="wikitable" style="text-align: center;" | ||
| + | |Strain | ||
| + | |[DNA] in ng/µL | ||
| + | |ratio | ||
| + | |- | ||
| + | |S38+pHyperspank 1 | ||
| + | |483.1 | ||
| + | |1.76 | ||
| + | |- | ||
| + | |S38+pHyperspank 2 | ||
| + | |281.9 | ||
| + | |1.67 | ||
| + | |} | ||
| + | |||
| + | I sent them for sequencing. | ||
| + | <br><br><br><br><br> | ||
Latest revision as of 15:21, 3 September 2011

Contents |
Cyrille
S24 + YFP-TetR:
- Prepare the 9 miniprep
- Replicate into glycerol
- PCR colony was done
YFP-TetR PstI-- 5re;oving the qdditionnal PstI site
- 5 Miniprep prepared
Competent cell preparation of MH1
Axel
extraction of ComS from 5 different colonies
same protocol as those from the 17th of august:
We are going to extract the ComS gene from the PY79 strain. Oligo has been designed for this purpose.
- Preparing the tube
Prepare 5 sterile tube with 20 µL of H2O for each colony Pick the colony using a pipetman woth a sterile tube. The pipetor is set at 3 µL for mixing
- Dissolving the primers
Resuspend the primers adding 10µL of water by nmol of primer. Final concentration at 100 mM. Add 1µL in 2 ml of water to get the 50 µM
- PCR mastermix
60 µL HF phusion buffer 6 µL dNTPs 15 µL of colony mix 6 µL Primer fwd at (50µM) 6 µL Primer rev at (50µM) 157 µL of H2O
Aliquot 49 µL in 5 PCR tubes Add 1 µL of phusion in each tube.
- PCR temperature
1 - 98°C - 15 min 2 - 98°C - 30 sec 3 - 70°C - gradient +-10 - 1 min 4 - 55°C - 1 min 5 - 72°C - 1 min 6 - Repeat 2-5 39 times 7 - 7°C forever
result of the gel: (biobricked ComS is about 160bp) ladder 100bp
Camille
From the overnight culture I did minipreps, and obtained :
| Strain | [DNA] in ng/µL | ratio |
| S38+pHyperspank 1 | 483.1 | 1.76 |
| S38+pHyperspank 2 | 281.9 | 1.67 |
I sent them for sequencing.
 "
"