Team:Freiburg/Notebook/9 September
From 2011.igem.org

Contents |
Commons
Testdigests
Investigators: Sophie
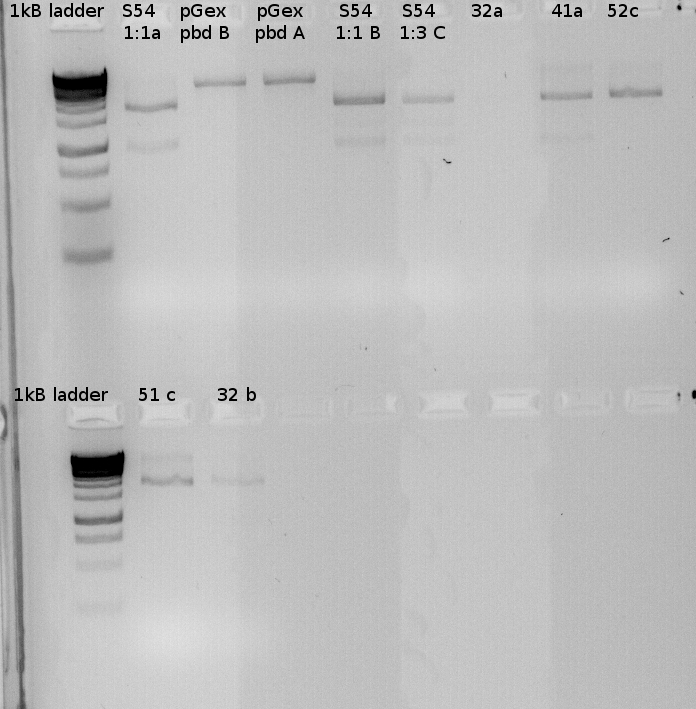
Testdigest of our various minipreps
green light receptor
digest for cloning PcpcG infront of CFP/YFP
Investigators:Julia
1. Digestion of PCR Product(PcpcG)
38µl PCR product
5µl BSA (10x)
5µl NEB buffer 4
1µl EcoRI
1µl SpeI
2.Digestion of CFP/YFP Vector
36,1/34 µl water
1.9µl DNA of YFP/ 3.7µl DNA of CFP = 500ng DNA
5µl BSA (10x)
5µl NEB buffer 4
1µl EcoRI
1µl XbaI
Incubation over night.
blue light receptor
Ligation
| Name: Rüdiger | Date: 09.09. |
| Continue from Date 05.09. Name Rüdiger
Experiment Digestion | |
| Project Name: Precipitator | |
Procedure
PCR tube:
total volume 20 μl
- add H2O (17 μl -X-Y-Z)
- add 2 μl Ligase Buffer 10x
- add Insert 1, Insert 2(when proceeding from 3A digestion use 2 μl of each)
- add Vector (20ng needed. When proceeding from 3A digestion use 2 μl)
- Add 1 μl T4-DNA Ligase
- Incubate 10-30 min at room temperature
- heat for 20 minutes at 80°C
- store at -20°C or directly proceed to transformation
| Name of part | Ratio Insert:Vector
= 3:1 or 1:1 | Volume (μl) | |
| X insert 1 | |||
| Y insert 2 | |||
| Z vector | |||
| H2O |
Documentation:
Why are you doing this experiment? Where are your parts stored? Name the parts for ligation etc.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Gibson assembly
| Name
Sandra, Sophie | Date:
26.07.2011 |
| Continue from Experiment (Date) from PCR (Lovtap 3000bp and Not-Gate 980bp) from today.
(Name) Sophie | |
| Project Name: Blue light receptor: Ligation of Lovtap and Not-Gate | |
Gibson-Assembly
1. Prepare 5X ISO buffer. Six ml of this buffer can be prepared by combining the following:
3 ml of 1 M Tris-HCl pH 7.5150 μl of 2 M MgCl260 μl of 100 mM dGTP 60 μl of 100 mM dATP 60 μl of 100 mM dTTP60 μl of 100 mM dCTP300 μl of 1 M DTT 1.5 g PEG-8000300 μl of 100 mM NADAdd water to 6 ml Aliquot 100 μl and store at -20 °C
2. Prepare an assembly master mixture. This can be prepared by combining the following:
320 μl 5X ISO buffer0.64 μl of 10 U/ μl T5 exo20 μl of 2 U/μl Phusion pol160 μl of 40 U/μl Taq ligAdd water to 1.2 ml
Aliquot 15 μl and store at -20 °C. This assembly mixture can be stored at -20 °C for at least one year. The enzymes remain active following at least 10 freeze-thaw cycles. This is ideal for the assembly of DNA molecules with 20-150 bp overlaps. For DNA molecules overlapping by larger than 150 bp, prepare the assembly mixture by using 3.2 μl of 10 U/ μl T5 exo.
3. Thaw a 15 μl assembly mixture aliquot and keep on ice until ready to be used.
4. Add 5 μl of DNA to be assembled to the master mixture. The DNA should be in equimolar amounts. Use 10-100 ng of each ~6 kb DNA fragment. For larger DNA segments, increasingly proportionate amounts of DNA should be added (e.g. 250 ng of each 150 kb DNA segment).
5. Incubate at 50 °C for 15 to 60 min (60 min is optimal).
6. If cloning is desired, electroporate 1 μl of the assembly reaction into 30 μl electrocompetent E. coli.
Documentation:
Why are you doing this experiment? Name the parts for the Gibson-Assembly.
| Parts for Gibson-Assembly: G-♥ and G-NOT |
How did you label your samples and where are they stored?
| Labelled G-♥-NOT and G-♥-NOT 50 |
PCR
| Name: Sophie
| Date: 9.9.11 |
| Continue from Experiment: Gibson (Date): 9.9.11
(Name): Sophie, Sandra | |
| Project Name: | |
PCR-Mixture for one Reaction:
For a 50 µl reaction use
| 32,5µl | H20 | Name |
| 10µl | 5x Phusion Buffer | of Primer |
| 2.5µl | Primer fw | P97 |
| 2.5µl | Primer dw | P100 |
| 1µl | dNTPs | of Template DNA |
| 1µl | DNA-Template | G-♥-NOT and G-♥-NOT 50 |
| 0.5 µl | Phusion (add in the end) |
What program do you use?
First 25 cycles: touchdown 69°C -0.4°C
next 5 cycles: touchdown 72°C -0.2°C
To confirm the PCR-Product has the correct size, load 2 µl of the sample onto an agarose-gel.
How did you label the PCR-Product, where is it stored and what do you do next?
Labeled: G-♥-NOT inserts a,b
stored in blue light box
Digestion
| Name: Sophie | Date: 9.9.11 |
| Continue from Date 9.9.11 Name: Sophie
Experiment: PCR | |
| Project Name: ♥-NOT in PR-vector | |
Procedure
- add H2O (38μl-DNA )
- 5 μl NEB4 buffer (stored at iGEM’s, -20°C)
- 5 μl 10x BSA (used 1:10 diluted sample stored at iGEM’s, -20°C)
- DNA (500 ng) (Vector:Insert ratio 1:3 in following Ligation)
- 1 μl restriction enzymes (stored at iGEM’s, -20°C)
- heat for 1-2 hours 37°C (6 hours if time)
- heat for 20 minutes 80°C (inactivation of enzymes)
- keep at 4°C if you cannot continue
Measured DNA-concentration with Nanodrop to calculate the volume of DNA to do the digestion:
| Sample Name | DNA concentration (μg/μl) |
| G-♥-NOT a,b | ~140 |
Restriction enzymes you need to cut the vector, insert1 and insert 2:
| Components | | Insert(μl) | |
| DNA (500ng) | |||
| BSA (10x) (5μl) | |||
| NEB4 Buffer (5μl) | |||
| Enzyme 1 (1μl) | Spe I | Nhe I | |
| Enzyme 2 (1μl) | Pst I | Pst I | |
| H2O (38 μl- DNA) | |||
| In total 50 μl | |||
Documentation:
Why are you doing this experiment? Where are the samples stored? Antibiotica resistance, vector used etc.
| The LOV-Repressor protein needs to be expressible
Samples stored in blue light box Vector used: all PR-vectors (D39- D44) |
Ligation
| Name: Sophie | Date: 9.9.11 |
| Continue from Date:9.9.11 Name: Sophie
Experiment: Digestion | |
| Project Name: G-♥-NOT in PR-vector | |
Procedure
PCR tube:
total volume 20 μl
- add H2O (17 μl -X-Y-Z)
- add 2 μl Ligase Buffer 10x
- add Insert 1, Insert 2(when proceeding from 3A digestion use 2 μl of each)
- add Vector (20ng needed. When proceeding from 3A digestion use 2 μl)
- Add 1 μl T4-DNA Ligase
- Incubate 10-30 min at room temperature
- heat for 20 minutes at 80°C
- store at -20°C or directly proceed to transformation
| Name of part | Ratio Insert:Vector
= 3:1 or 1:1 | Volume (μl) | |
| X insert 1 | G-♥-NOT insert a,b | ||
| Z vector | D39 -D44 | ||
| H2O |
Documentation:
Why are you doing this experiment? Where are your parts stored? Name the parts for ligation etc.
| Ligated parts labeled L39 -L44 (red pencil)
stored in blue light box |
Lysis cassette
Troubleshooting of the modified Lysis genes K124017
Investigators:Theo
The Ligated parts (M48+1 and M48+7) could not be transformed so it was decided to try again and wait until Monday for the sequencing.
In the meantime,
M48 is in an Amp Vector, part has to be sent to registry
The M48 stock was inoculated so that it could be mini-preped on Saturday
Precipitator
Transformation
| Name: Rüdiger | Date: 09.09. |
| Continue from Date 09.09. Name Rüdiger
Experiment Ligation | |
| Project Name: Precipitator | |
Procedure
- take cells from -80°C freezer and put them on ice! (every eppi contains about 400 μl cells)
- thaw cells on ice 20 minutes
- pipette 50 μl cells and 2 μl DNA into eppi still on ice!
- Incubate for 30 minutes on ice
- Heat at 42°C for 60 sec
- Incubate on ice for 5 minutes
- Add 200 μl LB Broth
- Incubate for 2 hours at 37°C (cells with lysis cassette at 30°C!!)
- Plate 50 μl and 200μl on two different LB/Agar plates with appropriate antibiotic resistance
Documentation:
Why are you doing this experiment? Name of the sample? Where are they stored? Name the vector with inserts, antibiotika resistance etc.
Had no C3 plates, took AMP plates only
Picked cells from yesterday's Transformation
4a/b 1-4
8a/b 1-4
9a/b 1-4
10a/b 1-4
NAME OF YOUR EXPERIMENT
Investigators: NAME
 "
"
 Contact
Contact