Team:Freiburg/Notebook/29 August
From 2011.igem.org

Contents |
green light receptor
PCR to amplify Green light Promotor PcpcG
Investigators:Julia PCR
| Name: Julia
| Date: 29.08.2011 |
| Project Name: Amplification of Green light Promotor | |
PCR-Mixture for one Reaction:
For a 50 µl reaction use
| 32,5µl | H20 | |
| 10µl | 5x Phusion Buffer | |
| 2.5µl | Primer fw | tatgaattcgcggccgcttctagaCCATTGTGCTTTTCTCTATCAACC |
| 2.5µl | Primer dw | tatctgcagcggccgctactagtaACTTAAAAGTTGTTTAATGTCCAGCC |
| 1µl | dNTPs | |
| 1µl | DNA-Template | original Synechocystis genome |
| 0.5 µl | Phusion |
What program do you use?
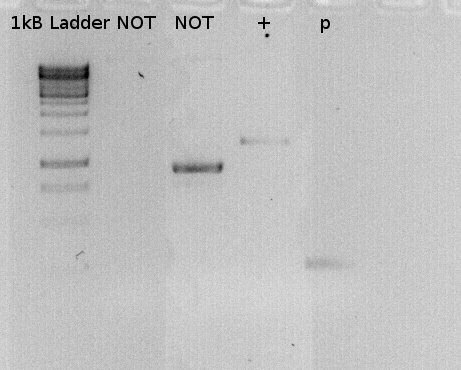
To confirm the PCR-Product has the correct size, load 2 µl of the sample onto an agarose-gel.
the lane labeled P, is the PCR product.Expected size: 264 bp
Testdigest of quickchanged CcaR and Precipitator
Investigators:Julia,with help of the team
3μl of the Miniprep were digested with EcoRI and PstI.

we are going to sequence the Precipitator samples 61a and b
(wrong labeling on the pic, its the 63 in the right corner)
62 b and f
63 c and d
and of the green light response regulator qCcaR 17b and 18a
blue light receptor
3A-assembly
Investigators: Sandra
Digestion I:
- Lovtap
- Not-Gate
- pSB1A3
- pSB1T3
stored in the blue light box.
Ligation
- ♥-A3 Not
- ♥-A3 nOt
- ♥-A3 noT
- ♥-T3 Not
- ♥-T3 nOt
- ♥-T3 noT
stored in the ligation box.
Digestion II:
Also digestion with new amplified vector
- pSB1C3
Lovtap was digested again this time also with DpnI. So for this ligation the ♥-PCR3A, digested with DpnI, and pSB1C3 (new amplified with PCR, already digested with DpnI) and Not, nOt, noT were used.
- ♥-C3 Not
- ♥-C3 nOt
- ♥-C3 noT
Transformation
Investigators: Sophie
transformation of:
- ♥-A3 Not
- ♥-A3 nOt
- ♥-A3 noT
- ♥-T3 Not
- ♥-T3 nOt
- ♥-T3 noT
Incubation over night at 37°C.
PCR
Investigator: Sophie
PCR
| Name:
| Date:
|
| Continue from Experiment: new
| |
| Project Name:
more NOT-gate with mutated restriction site (NHE I instead of Spe I) | |
PCR-Mixture for one Reaction:
For a 50 µl reaction use
| 32,5µl | H20 | Name |
| 10µl | 5x Phusion Buffer | of Primer |
| 2.5µl | Primer fw | P 24 (1:10) |
| 2.5µl | Primer dw | P 79 (1:10) |
| 1µl | dNTPs | of Template DNA |
| 1µl | DNA-Template |
M45 (Bba_Q04400) |
| 0.5 µl | Phusion (add in the end) |
The program we used: RT 2step (see last PCR of NOT-gate)

red light receptor
NAME OF YOUR EXPERIMENT
Investigators:NAME
Lysis cassette
NAME OF YOUR EXPERIMENT
Investigators:NAME
Precipitator
PCR
| Name:Rüdiger
| Date: 29.08 |
| Continue from Experiment PCR Rüdiger (Date)-22.08.
(Name) | |
| Project Name: Precipitator | |
PCR-Mixture for one Reaction:
For a 50 µl reaction use
| 32,5µl | H20 | Name |
| 10µl | 5x Phusion Buffer | of Primer |
| 2.5µl | Primer fw | P68Primer |
| 2.5µl | Primer dw | P70Primer |
| 1µl | dNTPs | of Template DNAPrimer |
| 1µl | DNA-Template | GST-6-P-1 a,b,c,d,ePrimer |
| 0.5 µl | Phusion (add in the end) |
What program do you use?
Annealing 60-70
To confirm the PCR-Product has the correct size, load 2 µl of the sample onto an agarose-gel.
How did you label the PCR-Product, where is it stored and what do you do next?
| Name | # | Template | Primer | Anneal. Temp. |
| For David Vector
Bam H1 +EcoR1 | 1 | Precipitator 3 | 87+88 | 65 |
| 2 | Precipitator 4_2 | 91+92 | 65 | |
| 3 | Precipitator 4 | 98+90 | 65 | |
| Gibson | 4 | pGEX-6-p-1 | 85+44 | 66 |
| 5 | pGEX-6-p-1 | 85+45 | 66 | |
| 6 | pGEX-6-p-1 | 85+46 | 66 | |
| Registry cloning | 7 | pGEX-6-p-1 | 85+48 | 66 |
| 8 | pGEX-6-p-1 | 48+84 | 66 | |
| Toolbox Gibson>GST-6-p-1 | 9 | Precipitator 2 | 81+73 | 67 |
| 10 | Precipitator 4_2 | 82+71 | 67 | |
| 11 | Precipitator 4 | 83+72 | 67 | |
| Control | 12 | Lambda DNA | Primers |
Testdigest of Precipitator in iGEM Vektor pSB1C3
Investigators: Julia and others
results see above under "green light receptor"
 "
"
 Contact
Contact